Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: There have not been large US-based studies of digital pitting scars (DPS) and digital ischemic ulcers (DIU) in systemic sclerosis. Utilizing the Collaborative National Quality and Efficacy Registry (CONQUER) cohort, we describe the prevalence, incidence, and quality of life impact of DPS and DIU and determine factors associated with the development of DPS or DIU.
Methods: CONQUER is a US-based, prospective, multi-center cohort of adults (age ≥18) with SSc who meet 2013 ACR/EULAR Classification Criteria and have a disease duration ≤5 years from first non-Raynaud’s symptom at enrollment. As of December 31st, 2024, 1113 participants with mean follow-up of 24 months were enrolled. At each visit, investigators noted the presence of DPS and DIU and collected demographic, clinical and laboratory variables. Multivariable logistic regression models were designed using directed acyclic graphs.
Results: The mean age at study entry was 52.5 years, 83% were female, 65% had diffuse cutaneous SSc and 64% had interstitial lung disease. At baseline, 20% had current DPS and 5% had DIU. Younger age at baseline, non-Hispanic Black or African American race, telangiectasias of face or lips, and positive Scl-70 antibody were associated with DPS at baseline (Table 1). Younger age was associated with DIU at baseline (Table 1). In contrast to the variables associated with DPS, positive Scl-70 antibody was associated with lower odds of DIU at baseline. During the first year in CONQUER, 14% developed new DPS and 15% developed new DIU. Analyses of demographic and SSc-related factors did not reveal significant predictors for time to development of first DPS or DIU (Figures 1 and 2). The presence of DIU, but not DPS, was associated with higher PROMIS-29 scales (worse symptom burden) from anxiety (OR 1.38, 95% CI 1.05 to 1.81), depression (OR 1.57, 95% CI 1.18 to 2.10), and pain (OR 1.40, 95% CI 1.04 to 1.89).
Conclusion: In this large, prospective US cohort of participants with early SSc, 14% developed DPS and 15% developed DIU in the first year of study. Non-Hispanic Black or African American race and younger age were associated with DPS, while younger age was associated with DIU. Although we did not identify significant predictors of incident DPS or DIU, DIU were associated with worse symptom burden. These findings underscore the importance of early vascular assessment in SSc and support further study to reduce ischemic complications and improve patient outcomes.
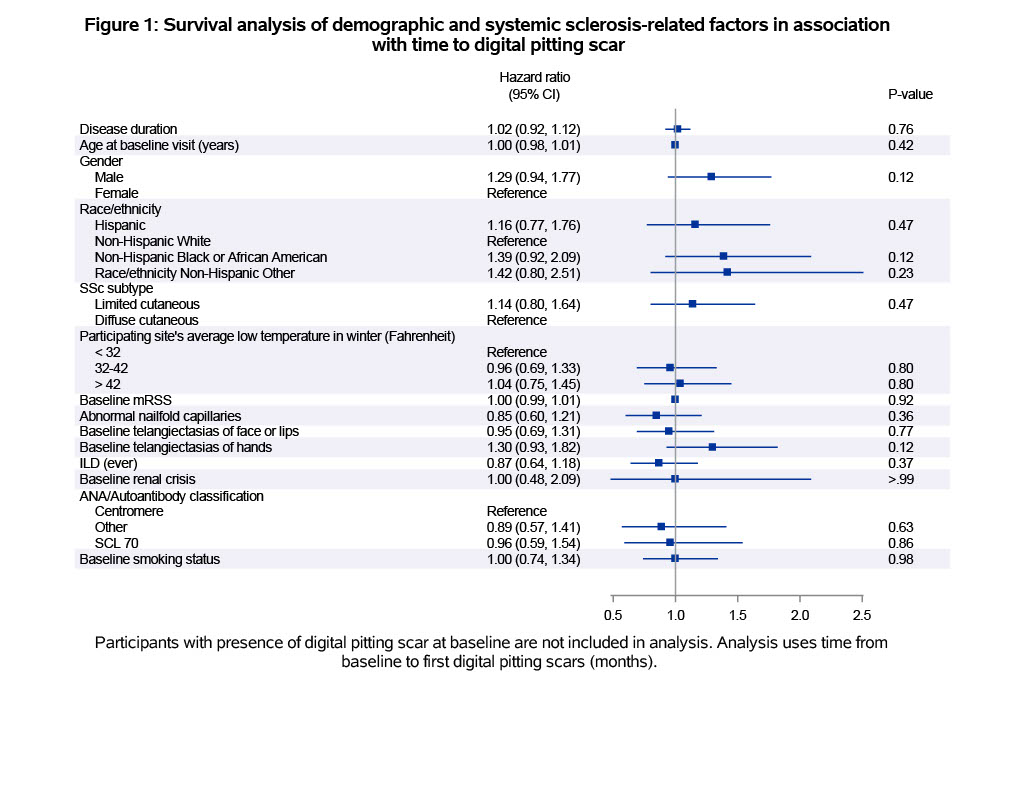 Table 1: Multivariable logistic regression of systemic sclerosis (SSc) characteristics and vascular risk factors with the presence of digital pitting scars and digital ischemic ulcers at baseline
Table 1: Multivariable logistic regression of systemic sclerosis (SSc) characteristics and vascular risk factors with the presence of digital pitting scars and digital ischemic ulcers at baseline
.jpg) Figure 1: Survival analysis of demographic and systemic sclerosis-related factors in association
Figure 1: Survival analysis of demographic and systemic sclerosis-related factors in association
with time to digital pitting scar
.jpg) Figure 2: Survival analysis of demographic and systemic sclerosis-related factors in association
Figure 2: Survival analysis of demographic and systemic sclerosis-related factors in association
with time to ischemic digital ulcers
To cite this abstract in AMA style:
Savoie M, Harding M, VanBuren J, Assassi S, Bernstein E, Chung L, Evnin L, Frech T, Gordon J, Hant F, Hummers L, Khanna D, Lakin K, Lebiedz-Odrobina D, Luo Y, Makol A, Mayes M, McMahan Z, Molitor J, Moore D, Richardson C, Sandorfi N, Shah A, Shah A, Skaug B, Steen V, Volkmann E, Zahn C, Castelino F. Risk factors for incident digital ischemic complications in systemic sclerosis in the Collaborative National Quality and Efficacy Registry (CONQUER) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/risk-factors-for-incident-digital-ischemic-complications-in-systemic-sclerosis-in-the-collaborative-national-quality-and-efficacy-registry-conquer/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-incident-digital-ischemic-complications-in-systemic-sclerosis-in-the-collaborative-national-quality-and-efficacy-registry-conquer/
