Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Although most vasoactive vasodilating drugs (VVDs) exert anti-fibrotic effects in pre-clinical studies, randomized controlled trials assessing their efficacy in systemic sclerosis-associated interstitial lung disease (SSc-ILD) have shown mixed results. Therefore, we assessed the impact of VVDs on functional progression of SSc-ILD in an observational, real-life setting.
Methods: We identified SSc patients with ILD diagnosed on computed tomography, follow-up pulmonary function tests (PFTs) and treatment data in the EUSTAR database and excluded those with pulmonary hypertension on right heart catheterization or with systolic pulmonary artery pressure on echocardiography >50mmHg at any timepoint. VVDs included endothelin-receptor antagonists, phosphodiesterase-5 inhibitors and prostanoids, administered for at least 3 months in the observation period. Five definitions of ILD progression within 12±3 months were tested: A) Forced vital capacity (FVC) decline≥10%, or 5-9% with diffusion capacity for carbon oxide (DLCO) decline ≥15% B) FVC decline ≥5% or DLCO decline ≥10%C) FVC decline ≥10%D) FVC decline ≥5%E) Worsening of NYHA class.Generalized estimated equation mixed models were performed separately for each ILD progression outcome. A Cox regression model with time-dependent variables was applied for the survival analysis, with mortality as outcome. Interaction terms were computed between VVDs and markers of peripheral/pulmonary vasculopathy (digital ulcers – DU, DLCO). All models were adjusted for known risk factors for ILD progression or mortality and ongoing immunosuppression.
Results: Among 5360 yearly visits of 1950 SSc-ILD patients, progression events ranged from 13 to 42%, according to the definition used (Table 1). Similarly, exposure to VVDs varied from 10 to 25% of the visits, with prostanoids being the most frequent.The interaction of prostanoids and DU was associated with progression A (p=0.057), B (p=0.028), C (p=0.006) and D (p=0.048). This translated into a protective effect of prostanoids against PFT decline in patients without DU, compared to patients with DU (Figures A-D). Additionally, prostanoids interacted significantly with DLCO, and this associated significantly with all progression definitions (A: p=0.022, B: p=0.003; C: p=0.001; D: p=0.023; E: p=0.010). Figures A-E show how higher DLCO values impact positively on the effectiveness of prostanoids. Combining all effects, prostanoids significantly associated with less PFT progression when used in patients without DU and with DLCO >70%, while DLCO values >100% would be needed for prostanoids to exert similar effects in DU patients. Over a median follow-up of 5.7 (2.8-9.9) years, 178/1950 (15.6%) SSc-ILD patients died. In the survival analysis, VVDs did not show any significant, independent impact on mortality.
Conclusion: Exposure to prostanoids is associated with lower risk of ILD progression in patients with mild vasculopathy (absence of DU, higher DLCO). Given our positive preliminary results on short-term progression, but the lack of independent impact on mortality, further studies are needed to confirm the beneficial effects of prostanoids on SSc-ILD.
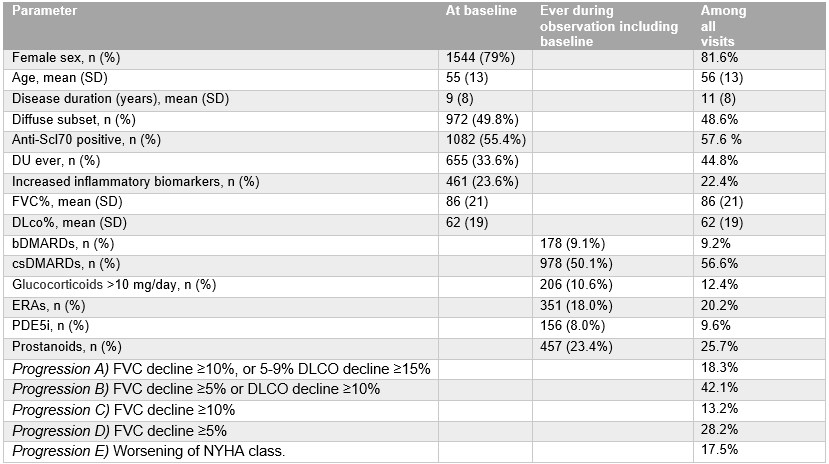 Table 1. Characteristics of the study population at baseline and across all visits.
Table 1. Characteristics of the study population at baseline and across all visits.
Biologic Disease-Modifying Anti-Rheumatic Drug (bDMARD), conventional synthetic Disease-Modifying Anti-Rheumatic Drugs (csDMARD), diffusing capacity for carbon monoxide (DLCO), digital ulcer (DU), endothelin-receptor antagonists (ERA), forced vital capacity (FVC), New York heart association (NYHA), phosphodiesterase-5 inhibitors (PDE5i)
To cite this abstract in AMA style:
sarbu a, Petelytska L, tofani l, Moroncini G, Balbir-Gurman A, zanatta e, Henes J, airò p, Matucci-Cerinic M, Gheorghiu A, marcoccia a, Anić b, Colic J, Furst D, Spierings j, Del Galdo F, Maurer B, Hoffmann-Vold A, Distler O, Bruni C. Vasodilation with Prostanoids Influences Progression of Systemic Sclerosis-Associated Interstitial Lung Disease: a EUSTAR Cohort Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/vasodilation-with-prostanoids-influences-progression-of-systemic-sclerosis-associated-interstitial-lung-disease-a-eustar-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vasodilation-with-prostanoids-influences-progression-of-systemic-sclerosis-associated-interstitial-lung-disease-a-eustar-cohort-study/

.jpg)