Session Information
Date: Monday, October 27, 2025
Title: (1306–1346) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Although genetic factors have been identified in the pathogenesis of RA, the concordance rate in monozygotic (MZ) twins is low, suggesting that other factors contribute to disease development. Further, the relative contribution of such non-genetic elements in identical twins has not been characterized. Here, we aimed to measure host and microbial biomarkers of RA by studying MZ twin pairs discordant for disease using a multi-omics approach.
Methods: Eight pairs of MZ twins discordant for RA (n=16; Table 1) were enrolled in the United States (US). Gut microbiome was assessed using shotgun metagenomic sequencing. Autoantibodies, cytokines, and other plasma proteins were measured in both plasma and feces. Levels of short-chain fatty acids (SCFA) from serum and feces were quantified using gas chromatography mass spectrometry (GC-MS). A metagenomic dataset from a geographically distinct cohort of MZ twins discordant for RA (n=14, Table 2) from TwinsUK was used to validate the findings.
Results: While overall microbiome diversity and composition did not significantly differ between twin pairs in either cohort, we observed a decrease in Blautia faecis, a SCFA producer, in RA affected US twins (Fig. 1 A). Affected US twins had higher concentrations of both fecal and plasma citrullinated and non-citrullinated autoantibodies, as well as significantly lower concentrations of fecal butyrate and propionate (Fig. 1 B,C). TwinsUK RA discordant pairs showed comparable differences using metagenomic-based taxa- and pathways-features. SCFA producing bacteria and pathways were depleted in TwinsUK RA affected twins (Fig 1 D,E).
Conclusion: Multi-omic biomarkers differentiate MZ twins discordant for RA. Blautia faecis, which is associated with SCFA production and reduced inflammatory cytokine expression, was decreased in US affected twins. Similarly, SCFA, which are known to have immune modulatory effects, were decreased in affected twins, suggesting further bi-directional interactions between inflammation at the gut barrier and disease state. We observed similar changes in SCFA producing taxa between unaffected and affected twins in a geographically distinct twin cohort. If confirmed in larger cohorts, exhaustive multi-omic approaches may improve our understanding of RA pathogenesis. Future work should focus on the potential for SCFA in diagnostics and therapeutics.
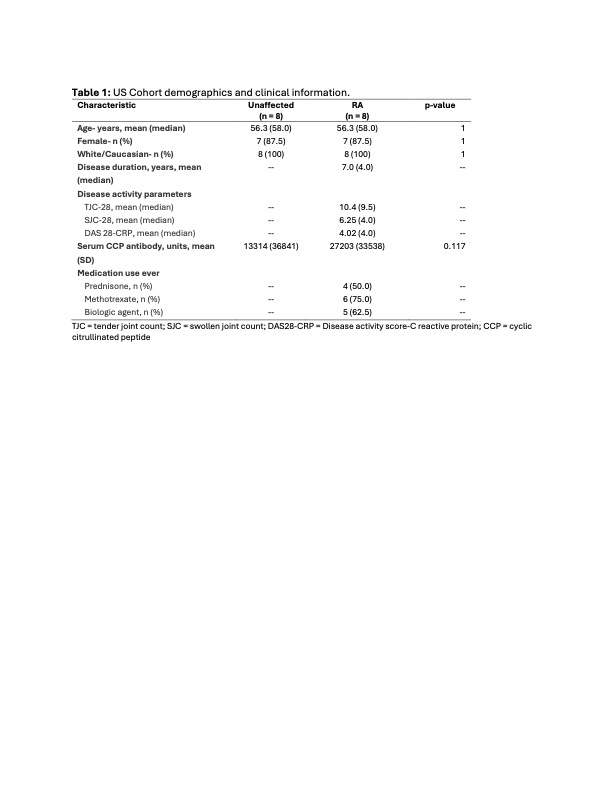 Table 1: US Cohort demographics and clinical information.
Table 1: US Cohort demographics and clinical information.
.jpg) Table 2. TwinsUK sample demographics and clinical information.
Table 2. TwinsUK sample demographics and clinical information.
.jpg) Figure 1. Gut bacterial composition, predicted functional pathways, and fecal SCFA concentration in affected and unaffected twins in US and UK cohorts. (A-C are US cohort; D-E are UK cohort.) (A) Boxplot showing distribution of B. faecis abundance in unaffected and affected US twins. Fecal concentration of butyrate (B) and propionate (C) in unaffected and affected US twins. (D) Linear discriminant analysis Effect Size (LEfSe) showing differential species in unaffected twins compared to affected twins in UK cohort. Gemmiger and Faecalicatena are SCFA producers. (E) Barplot of Wilcoxon effect sizes for differential pathways in UK cohort. *p < 0.05, **p < 0.01.
Figure 1. Gut bacterial composition, predicted functional pathways, and fecal SCFA concentration in affected and unaffected twins in US and UK cohorts. (A-C are US cohort; D-E are UK cohort.) (A) Boxplot showing distribution of B. faecis abundance in unaffected and affected US twins. Fecal concentration of butyrate (B) and propionate (C) in unaffected and affected US twins. (D) Linear discriminant analysis Effect Size (LEfSe) showing differential species in unaffected twins compared to affected twins in UK cohort. Gemmiger and Faecalicatena are SCFA producers. (E) Barplot of Wilcoxon effect sizes for differential pathways in UK cohort. *p < 0.05, **p < 0.01.
To cite this abstract in AMA style:
Blank R, Bu K, Zhang X, Chen W, Cunningham I, sokolove j, Lahey L, Heguy A, Medina R, Ubeda C, Nayak R, Hu J, Cantor A, Lee J, Williams F, Clemente J, Scher J. Short-Chain Fatty Acids and Their Gut Microbial Metabolic Pathways Distinguish Rheumatoid Arthritis in Discordant Monozygotic Twins [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/short-chain-fatty-acids-and-their-gut-microbial-metabolic-pathways-distinguish-rheumatoid-arthritis-in-discordant-monozygotic-twins/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-chain-fatty-acids-and-their-gut-microbial-metabolic-pathways-distinguish-rheumatoid-arthritis-in-discordant-monozygotic-twins/
