Session Information
Date: Monday, October 27, 2025
Title: (1306–1346) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Cardiovascular disease (CVD) remains the leading cause of death in rheumatoid arthritis (RA), driven primarily by atherosclerotic CVD (ASCVD) and heart failure (HF). Malondialdehyde-acetaldehyde (MAA) adducts are highly immunogenic post-translational modifications (PTM) that result from oxidative stress. MAA adducts are enriched in serum and synovial fluid from patients with RA, as well as the serum and atheromatous lesions in non-RA patients with coronary artery disease. Given the ongoing need to improve CVD risk stratification and management in RA, our objective was to evaluate the associations of protein-specific anti-MAA antibodies with incident ASCVD and HF outcomes in RA.
Methods: We conducted a cohort study among participants in the multicenter Veterans Affairs RA registry. Antibodies to MAA-modified-albumin, -collagen, -fibrinogen, and -vimentin (IgA, IgG, IgM isotypes) were measured by ELISA on banked serum from registry enrollment. Antibody concentrations were log transformed and standardized (per 1 S.D.), categorized into quartiles, and dichotomized at the highest quartile (high vs. low). Incident ASCVD (fatal or non-fatal myocardial infarction or stroke) and HF (HF hospitalization or HF-related death) were identified using validated administrative algorithms in linked VA, Medicare, and National Death Index data. Patients were followed from enrollment to incident CVD, death, or end of study period (12/2022). The associations of anti-MAA antibodies with incident ASCVD and HF were tested separately in multivariable Cox regression models adjusted for age, sex, race, smoking status, body mass index, comorbidities (hypertension, hyperlipidemia, diabetes, chronic lung disease), seropositivity (for RF and/or anti-CCP), and DAS28.
Results: There were 3,572 participants included in this study (87% male, mean age 64 years, mean DAS28 3.5). Among those without prior ASCVD and HF, we observed 398 incident ASCVD (20.9 events per 1000 person years [PY] over 23,056 PY follow-up) and 327 incident HF events (14.2 per 1000 PY over 19,049 PY follow-up). High values (top vs. lower quartiles) of anti-MAA-vimentin were significantly associated with incident ASCVD for IgG (aHR 1.42, 95% CI 1.13-1.79), IgM (1.27, 1.01-1.60), and IgA isotypes (1.30, 1.03-1.64; Table 1). A dose-dependent relationship was observed between IgG anti-MAA-vimentin and incident ASCVD (p< 0.01 for trend across all 4 quartiles; Figure 1). In analyses of all 4 quartiles, higher IgM anti-MAA-vimentin tended to be associated with incident HF (p for trend 0.04; Figure 1); findings were not significant for analyses categorized as high vs. low (aHR 1.25, 0.97-1.61; Table 1). Other protein-specific anti-MAA antibodies were not associated with ASCVD or HF.
Conclusion: Antibodies against MAA-modified vimentin were independently associated with incident ASCVD in a large, prospective RA cohort. Vimentin, an extracellular matrix protein, commonly undergoes PTM during RA development and MAA is over-expressed in myocardial tissues in animal models of RA. These data support the hypothesis that vimentin modification may serve as a unique driver of ASCVD in RA, and anti-MAA-vimentin antibodies may facilitate CVD risk stratification in RA.
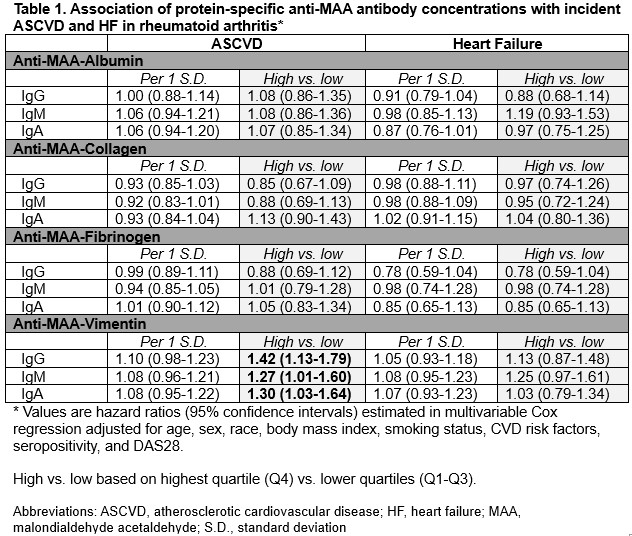 Table 1. Association of anti-MAA antibodies with ASCVD and HF in RA
Table 1. Association of anti-MAA antibodies with ASCVD and HF in RA
.jpg) Figure 1. Association of anti-MAA vimentin quartiles with ASCVD and HF in RA
Figure 1. Association of anti-MAA vimentin quartiles with ASCVD and HF in RA
To cite this abstract in AMA style:
King J, Duryee M, Roul P, Wysham K, Cannon G, Kunkel G, Richards J, Smith I, Wallace B, Kerr G, Reimold A, Schwab P, Anderson D, Zhou W, Baker J, Mikuls T, England B, Johnson T. Protein-Specific Anti-Malondialdehyde-Acetaldehyde Antibodies and Cardiovascular Disease Outcomes in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/protein-specific-anti-malondialdehyde-acetaldehyde-antibodies-and-cardiovascular-disease-outcomes-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/protein-specific-anti-malondialdehyde-acetaldehyde-antibodies-and-cardiovascular-disease-outcomes-in-rheumatoid-arthritis/
