Session Information
Date: Monday, October 27, 2025
Title: (1191–1220) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Dermatomyositis (DM) is a rare autoimmune disease characterized by cutaneous manifestations and proximal muscle inflammation.1,2 Notably, the clinical course of muscular and cutaneous disease in DM is highly variable. Some patients may exhibit predominant muscle disease while up to 20% of patients are categorized as clinically amyopathic and present with skin manifestations in the absence of muscle disease.3 However, most clinical trials require both muscle and skin involvement to be eligible.4 Our center has observed that even DM patients who are not amyopathic at presentation will often have improvement in muscle activity before skin activity, which prevents patients from being eligible for clinical trials given the lack of both muscular and skin involvement. Our study aims to characterize the clinical trajectory of muscular and cutaneous disease activity in patients with DM.
Methods: Patients with DM satisfying the ACR/EULAR 2017 classification criteria were prospectively enrolled between 2019 and 2025 at a large United States tertiary academic medical center. Patients included in this study were adult patients (≥18 years old at DM symptom onset), had clinical active disease as defined by Manual Muscle Testing-8 (MMT-8) < 142 and Cutaneous Dermatomyositis Disease Area and Severity Index-Activity (CDASI-A) >6 and at least 3 consecutive visits over 24 months (Figure 1). Demographic data, DM disease history, serologic status and treatment history were obtained through prospective discrete data capture at each clinical visit. Outcomes included the percentage of patients who achieved improvement of their cutaneous and muscular disease as measured by the CDASI-A (decrease in 40%) and MMT-8 scores (near normalization of strength, ≥142).5
Results: 20 patients with adult DM met the inclusion criteria (95% female, 75% White; mean age at DM diagnosis 47) with demographics outlined in Table 1. The baseline visit was on average five years after DM onset. Baseline median CDASI activity was 9 (IQR 8.0-13.5) and baseline median MMT-8 was 132 (IQR 122-137). From the baseline visit to the second visit (median 5 months after baseline), the median CDASI-A improved from 9 to 8 whereas the MMT-8 improved from 132 to 144. Only 7 (35%) patients experienced cutaneous improvement, whereas 11 (55%) patients experienced strength improvement. By the 3rd visit (median 10 months after baseline) median CDASI-A improved to 5 and MMT-8 improved to 146. At this time, 15 (75%) patients achieved cutaneous improvement, and 16 (80%) patients achieved muscular improvement (Figure 2). The median time to improvement for cutaneous disease was 6.5 months (IQR 5-11) whereas median time to improvement for muscular strength was 5 months (IQR 3-9.5).
Conclusion: At our U.S. tertiary academic medical center, most adult dermatomyositis (DM) patients present with relatively preserved muscle strength but more active skin disease. As a result, many are ineligible for clinical trials that require significant muscle weakness. Longitudinal data indicate that muscle symptoms often improve before skin symptoms, and the delay between disease onset and referral to a tertiary center may further hinder clinical trial recruitment.
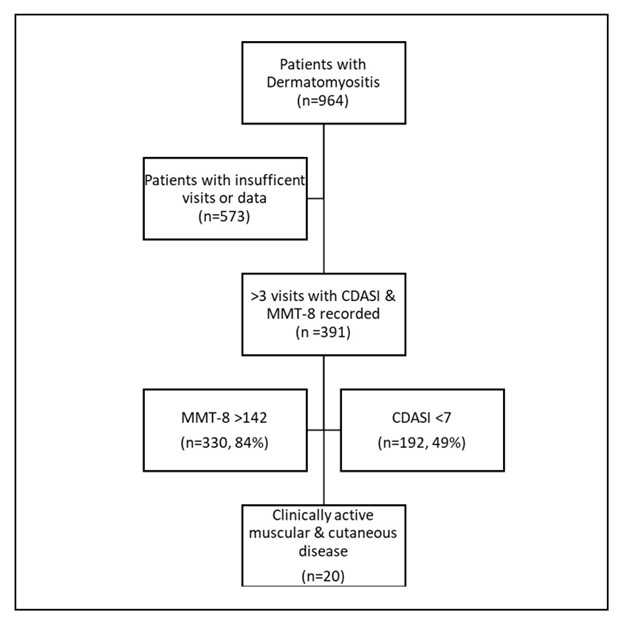 Figure 1. Of the 391 DM eligible cohort, 330 (84%) of patients did not meet muscle criteria whereas 199 (51%) met cutaneous criteria, suggesting most patients are not eligible for many clinical trials due to lack of weakness.
Figure 1. Of the 391 DM eligible cohort, 330 (84%) of patients did not meet muscle criteria whereas 199 (51%) met cutaneous criteria, suggesting most patients are not eligible for many clinical trials due to lack of weakness.
.jpg) Figure 2. Cutaneous vs Muscular Disease Improvement Over Time
Figure 2. Cutaneous vs Muscular Disease Improvement Over Time
.jpg) Table 1. Demographic and Disease Characteristics
Table 1. Demographic and Disease Characteristics
To cite this abstract in AMA style:
Valencia J, Kelly W, Tackett S, Finney A, Albayda J, Paik J, Christopher-Stine L, Mecoli C. Cutaneous and Muscular Disease Trajectories in Adult Patients with Dermatomyositis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/cutaneous-and-muscular-disease-trajectories-in-adult-patients-with-dermatomyositis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cutaneous-and-muscular-disease-trajectories-in-adult-patients-with-dermatomyositis/
