Session Information
Date: Monday, October 27, 2025
Title: (1123–1146) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Gout is an inflammatory arthritis characterized by acute attacks, tophi formation, and bone destruction triggered by inflammatory responses to monosodium urate (MSU) crystals. Natural killer (NK) cells are key effectors of the innate immune system that recognize damage-associated molecular patterns (DAMPs) to initiate and amplify inflammatory responses. While MSU crystals deposited in the synovium may act as DAMPs and potentially activate NK cells, the role of these cells in gout pathophysiology remains unclear.
Methods: Peripheral blood and synovial fluid samples were collected from patients with gout and healthy controls (HCs). Flow cytometry was performed to analyze NK cells for their activation markers (CD69, PD-1, TIM-3), apoptosis (Annexin V), and cytokine/chemokine expression (IFN-γ, TNF-α, CCR2, CCR6, CXCR3, and CXCR4). NK cells were stimulated with MSU crystals and sorted by FACS sorter, while monocytes were isolated from PBMC using MACS. To evaluate osteoclastogenesis, MSU-stimulated NK cells and monocytes were cocultured in the presence of M-CSF, followed by TRAP staining. The expression of RANKL in MSU-stimulated NK cells was assessed using both flow cytometry and RT-PCR.
Results: The frequency of NK cells was decreased in the peripheral blood of gout patients, with intercritical stage patients showing a statistically significant reduction compared to HCs. These NK cells exhibited elevated annexin V expression, indicating late-stage apoptosis. Under PMA-IM stimulation, NK cells from both gout patients and HCs produced comparable levels of IFN-γ and TNF-α. In synovial fluid, the frequency of NK cells was significantly lower than in peripheral blood, but their CD69 and annexin V expression levels were higher. Chemokine receptor expression, including CCR2, CCR6, CXCR3, and CXCR4, tend to be elevated in synovial fluid-derived NK cells. Furthermore, NK cells stimulated with MSU crystals demonstrated increased CD69 and annexin V expression, suggesting activation by MSU. Notably, MSU-activated NK cells promoted osteoclastogenesis in both direct and indirect manners, as shown in co-culture experiments with or without transwell systems. These cells also exhibited elevated RANKL expression, confirmed by both flow cytometry and RT-PCR.
Conclusion: NK cells are numerically reduced and may undergo late-stage apoptosis in the peripheral blood of gout patients. In synovial fluid, NK cells show elevated expression of activation markers and chemokine receptors despite their decreased numbers, suggesting their apoptosis or migration from peripheral tissues to the synovium. MSU crystals activate NK cells, which in turn promote osteoclastogenesis, partly through increased RANKL expression. These findings suggest that NK cells play a key role in amplifying synovial inflammation and contributing to bone destruction in gout.
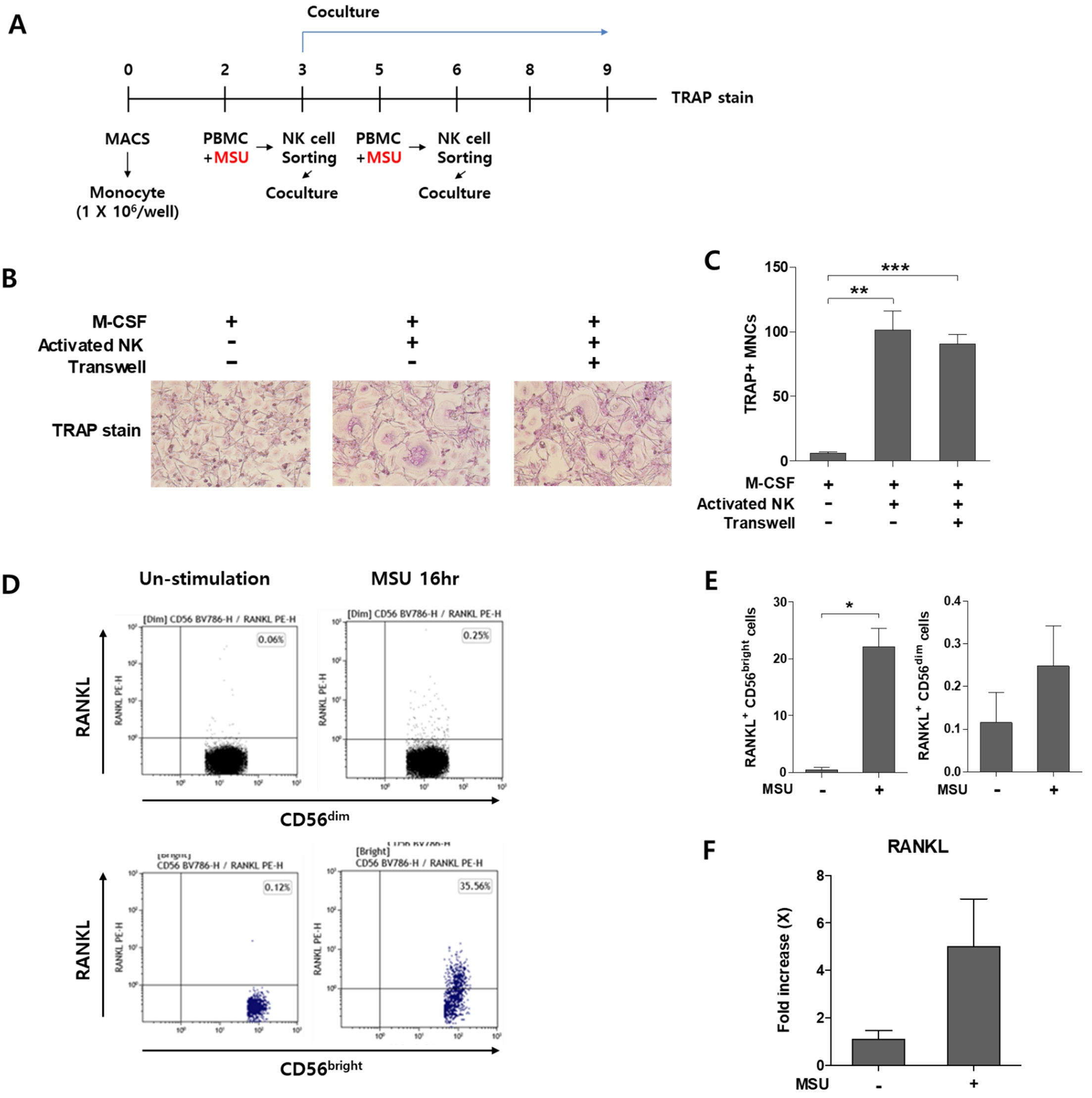 MSU-activated NK cells can induce osteoclastogenesis and express RANKL
MSU-activated NK cells can induce osteoclastogenesis and express RANKL
(A) Scheme of the experiment (B-C) TRAP stain of MSU-Activated NK cells (D-E) Representative gating and Frequency of RANKL in CD56dim and CD56bright NK cells with or without MSU crystal using flow cytometry (F) RANKL expression of NK cells with or without MSU crystal in RT-PCR
To cite this abstract in AMA style:
Park K, Cho Y, Jin H, Jeong H, Choi S, Kang J, Park D, Kim T, Lee S, Park Y. Numerically Reduced but MSU Crystal-Activated NK Cells Promote Osteoclastogenesis in Gout [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/numerically-reduced-but-msu-crystal-activated-nk-cells-promote-osteoclastogenesis-in-gout/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/numerically-reduced-but-msu-crystal-activated-nk-cells-promote-osteoclastogenesis-in-gout/
