Session Information
Date: Monday, October 27, 2025
Title: (1123–1146) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Uncontrolled gout (UG) occurs when serum uric acid (sUA) levels remain persistently elevated despite use of oral urate-lowering therapies and can result in progressively painful gout flares (Dalbeth et al. Nat Rev Dis Primers 2019). Nanoencapsulated sirolimus plus pegadricase (NASP, formerly SEL-212) is a novel investigational therapy delivered every 4 weeks as a sequential, two-component infusion consisting of targeted nanoencapsulated sirolimus and pegadricase, a pegylated uricase (Figure 1A). Here, we report outcomes through 48 weeks in a subset of patients (pts) in the DISSOLVE I study who received 6 doses of NASP or placebo (PBO) during the 24-week main study period.
Methods: This is a post-hoc analysis of the DISSOLVE I (NCT04513366) trial that randomized pts 1:1:1 to one of two doses of NASP (high-dose [HD] or low-dose [LD]) or PBO. After each study drug administration, 4 weeks of observation followed for a total of 6 treatment periods during the main study. Pts could enter a 24-week double-blind extension after the main study period (Figure 1B). Evaluated endpoints included reductions in sUA, gout flares, and pts meeting stopping rule criteria (Kivitz et al. EULAR 2024, POS0244). Safety was monitored throughout.
Results: Of the 38 pts receiving HD NASP, 37 LD NASP and 37 PBO in DISSOLVE I, 19, 16 and 25, respectively, received all 6 doses of study drug during main study period, and all (100%) entered the extension phase. At baseline, mean (standard deviation [SD]) sUA was 8.4 (1.45) mg/dL in HD NASP, 8.7 (1.08) mg/dL for LD NASP, and 8.3 (1.43) mg/dL for PBO. Immediately after the first dose, mean (SD) sUA dropped to 0.2 (0.00) mg/dL with HD and LD NASP, while remaining stable for PBO at 8.2 (1.36) mg/dL. sUA was maintained low at the end of the first treatment period (Week 4) with NASP (mean (SD) sUA: HD, 1.0 (2.5) mg/dL; LD, 0.2 (0.13) mg/dL) while remaining stable with PBO at 8.4 (1.28) mg/dL. Low sUA were maintained throughout treatment with NASP (Weeks 24: HD, 1.8 (3.29) mg/dL; LD, 1.7 (2.41) mg/dL; Week 48: HD, n=16, 2.4 (3.67) mg/dL; LD, n=12, 1.3 (2.80) mg/dL). In PBO-treated pts, sUA remained consistent at 8.6 (1.43) mg/dL and 8.1 (1.94) mg/dL at Weeks 24 and 48 (n=22), respectively. The proportion of pts with gout flares decreased with NASP treatment over the study period. During Weeks 1–4, flares occurred in 3/19 (15.8%) pts on HD NASP, 7/16 (43.8%) on LD NASP, and 7/25 (28.0%) on PBO. During Weeks 21–24, flares occurred in 1/19 (5.3%) pts on HD NASP, 2/16 (12.5%) on LD NASP, and remained at 7/25 (28.0%) on PBO and during Weeks 45–48, 0/16 (0%) pts on HD NASP and 1/13 (7.7%) on LD NASP experienced flares vs 5/22 (22.7%) on PBO. In the extension phase, no new safety signals were reported, and no patients experienced an infusion reaction within 1 hour. Only 1 pt (3.7%) in each NASP dose group discontinued treatment due to meeting the stopping rule criteria.
Conclusion: These data support the long-term durability of NASP as shown by sustained sUA reductions and decreased gout flares over time, with 100% on HD NASP and 92.3% on LD NASP being flare-free at the end of the extension phase. NASP was generally well tolerated with low discontinuation rates during the extension phase. Overall, NASP led to improvements in key clinical manifestations of UG.
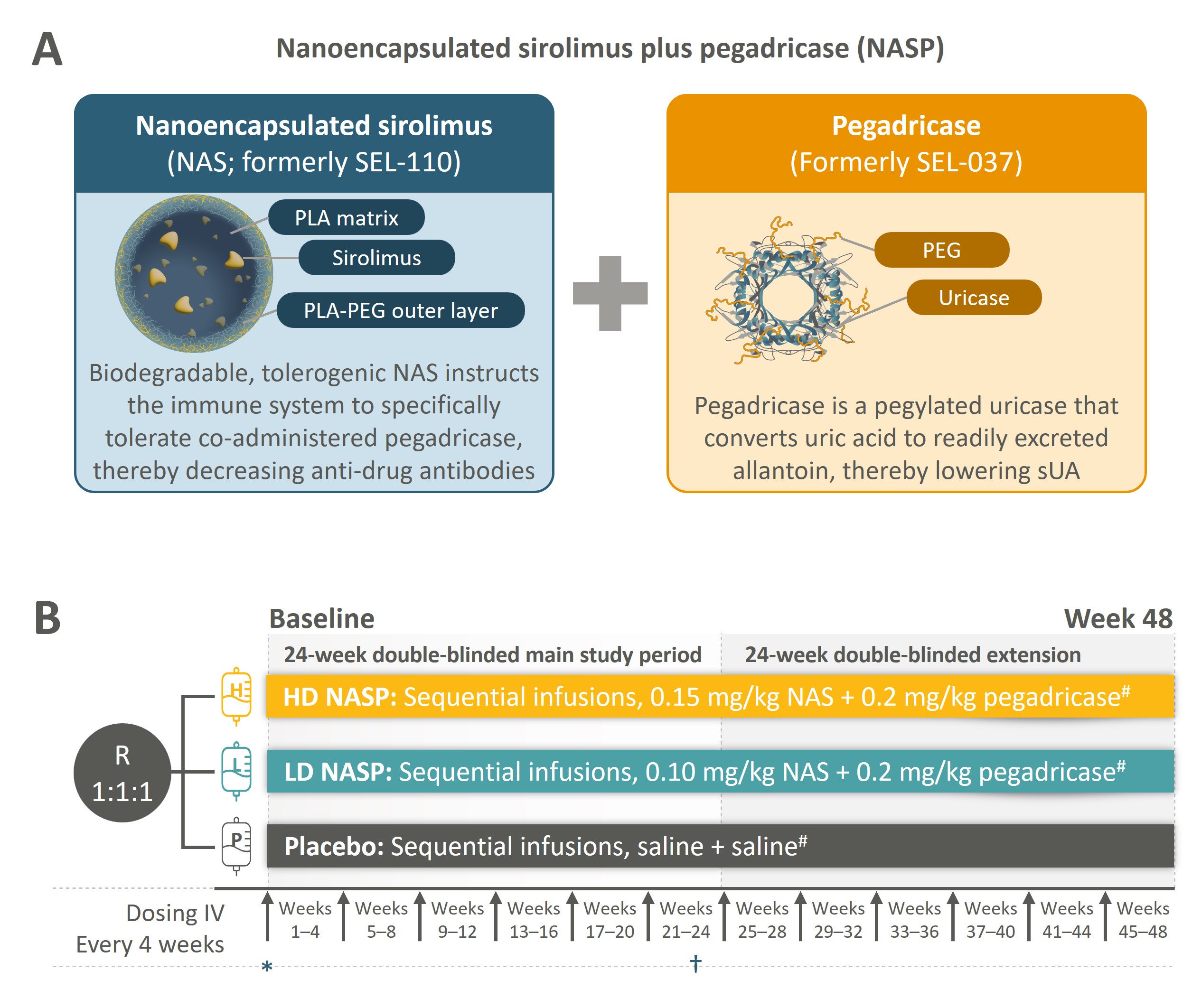 Figure 1. Schematic representation of nanoencapsulated sirolimus plus pegadricase (NASP) composition (A) and DISSOLVE I (NCT04513366) study design (B). #Treatment was discontinued if the stopping rule was met: sUA < 2.0 mg/dL 1 hour after infusion of the second component of the study drug during week 1 AND either sUA >1.0 mg/dL at the end of week 3 OR sUA >6.0 mg/dL at the end of any subsequent week (i.e. week 7, 11, 15, 19, 23, 27, 31, 35, 39, or 43).
Figure 1. Schematic representation of nanoencapsulated sirolimus plus pegadricase (NASP) composition (A) and DISSOLVE I (NCT04513366) study design (B). #Treatment was discontinued if the stopping rule was met: sUA < 2.0 mg/dL 1 hour after infusion of the second component of the study drug during week 1 AND either sUA >1.0 mg/dL at the end of week 3 OR sUA >6.0 mg/dL at the end of any subsequent week (i.e. week 7, 11, 15, 19, 23, 27, 31, 35, 39, or 43).
Arrows at each interval indicate the timing of drug administration.
*sUA measured 1 hour after completing infusion of second study drug. †Evaluation of primary endpoint, defined as the percentage of patients who achieved and maintained sUA < 6 mg/dL for ≥80% of the time during Weeks 21–24. Evaluation of sUA during Weeks 21–24 for the primary endpoint was done before study drug infusion, 1 hour after completion of study drug infusion, and at the end of each week during Weeks 21–24.
HD, high-dose; IV, intravenous; LD, low-dose; NAS, nanoencapsulated sirolimus; NASP, nanoencapsulated sirolimus plus pegadricase; PEG, polyethylene glycol; PLA, polylactic acid; R, randomization; sUA, serum uric acid.
To cite this abstract in AMA style:
Kivitz A, Singhal A, Patel A, Azeem R, Peace B, Desai B, Baraf H. Nanoencapsulated Sirolimus plus Pegadricase (NASP) Demonstrates Long Term Efficacy and Safety in Patients with Uncontrolled Gout: Results from the 24-week Double-blind Extension of the Phase 3 DISSOLVE I Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/nanoencapsulated-sirolimus-plus-pegadricase-nasp-demonstrates-long-term-efficacy-and-safety-in-patients-with-uncontrolled-gout-results-from-the-24-week-double-blind-extension-of-the-phase-3-dissolv/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nanoencapsulated-sirolimus-plus-pegadricase-nasp-demonstrates-long-term-efficacy-and-safety-in-patients-with-uncontrolled-gout-results-from-the-24-week-double-blind-extension-of-the-phase-3-dissolv/
