Session Information
Date: Monday, October 27, 2025
Title: (0978–1006) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Herpes zoster (HZ) is a common infection, particularly in older adults and women, and can lead to complications like neuropathic pain. The risk is higher in immunosuppressed individuals. Shingrix™, a recombinant subunit vaccine, has proven effective in reducing HZ risk by stimulating robust CD4 and CD8 T-cell responses. However, data on its immunogenicity in patients with immune-mediated inflammatory diseases (IMIDs) remains limited. This study aimed to evaluate B-cell and T-cell immune responses to the recombinant HZ vaccine in IMID patients treated with JAK inhibitors (JAK-i), anti-TNF therapies, or methotrexate (MTX), and to identify factors associated with reduced vaccine-induced immunity.
Methods: This study investigated both humoral and cellular immune responses following a two-dose regimen of the recombinant inactivated vaccine in 131 patients with IMIDs treated with JAK inhibitors, anti-TNF therapy, or MTX. The results were compared to those of 27 healthy controls matched for age and sex. Cellular immune responses were assessed through CD4+ (Th1) and CD8+ (cytotoxic) T-cell activation, while VZV-specific IgG antibody levels were used to evaluate humoral responses.
Results: Among 157 participants (mean age 58.2 years), JAK-i users had significantly lower immune responses. Seroconversion was 86% in JAK-i patients, compared to 97% in anti-TNF/MTX and 100% in controls (P = 0.03). VZV IgG titers were significantly lower in the JAK-i group (mean 1842 U/mL) versus anti-TNF/MTX and controls (both ~3,000 U/mL, P < 0.0001). Only 28% of JAK-i patients had a full CD4+ Th1 response (vs. 74% anti-TNF/MTX, 93% controls), and CD8+ responses were similarly reduced (29% vs. 42% and 85%, respectively). Immunogenicity was negatively associated with cumulative MTX and glucocorticoid use, prior DMARDs, baricitinib and tofacitinib treatment, and longer JAK-i exposure. A positive correlation was seen between CD4+ and CD8+ responses (β = 0.36, P = 0.0002).
Conclusion: IMID patients treated with JAK inhibitors exhibit significantly reduced humoral and cellular immune responses to the HZ recombinant vaccine, suggesting lower protection against VZV reactivation. While some reduction was seen in the anti-TNF/MTX group, their immune responses remained relatively preserved. The findings underscore the impact of JAK inhibitors and cumulative immunosuppression on vaccine efficacy in this population.
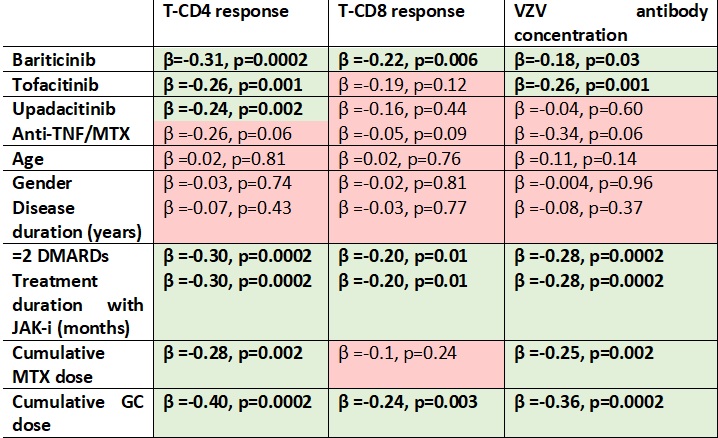 Correlations between T-CD4 response, T-CD8 response, and VZV antibody concentration with JAK-I, anti-TNF/MTX, age, gender, disease duration, treatment with ≥2 DMARDs, treatment duration with JAK inhibitors, and cumulative doses of MTX and GC. Linear logistic regression coefficients (β) and p-values are presented, with significant parameters highlighted in bold.
Correlations between T-CD4 response, T-CD8 response, and VZV antibody concentration with JAK-I, anti-TNF/MTX, age, gender, disease duration, treatment with ≥2 DMARDs, treatment duration with JAK inhibitors, and cumulative doses of MTX and GC. Linear logistic regression coefficients (β) and p-values are presented, with significant parameters highlighted in bold.
.jpg) Comparison of post-vaccination concentrations of granzyme A (2a), granzyme B (2b), TNF (2c) and IL-6 (2d) in healthy controls, IMRD JAK-I and IMRD anti-TNF/MTX patients. Higher levels of granzyme B (p=0.03), IL-6 (p=0.005) and IL-2 (p < 0.0001) were observed in patients treated with anti-TNF/MTX as compared with baricitinib, tofacitinib and upadacitinib. Box-and-whiskers, 5–95 percentile, ANOVA test (3A–3D). P values are indicated above each graph.
Comparison of post-vaccination concentrations of granzyme A (2a), granzyme B (2b), TNF (2c) and IL-6 (2d) in healthy controls, IMRD JAK-I and IMRD anti-TNF/MTX patients. Higher levels of granzyme B (p=0.03), IL-6 (p=0.005) and IL-2 (p < 0.0001) were observed in patients treated with anti-TNF/MTX as compared with baricitinib, tofacitinib and upadacitinib. Box-and-whiskers, 5–95 percentile, ANOVA test (3A–3D). P values are indicated above each graph.
To cite this abstract in AMA style:
Sieiro c, García Herrero J, Ordas Martínez J, Álvarez Castro C, López Robles A, Colindres R, Sahagún A, Ruiz de Morales J. Impact of Immunosuppressive Therapies on Shingrix Vaccine Response in Immune-Mediated Inflammatory Diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-of-immunosuppressive-therapies-on-shingrix-vaccine-response-in-immune-mediated-inflammatory-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-immunosuppressive-therapies-on-shingrix-vaccine-response-in-immune-mediated-inflammatory-diseases/
