Session Information
Date: Monday, October 27, 2025
Title: (0955–0977) Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The identification of effective and safe anti-fibrotic agents is a critical unmet need in systemic sclerosis (SSc). Although fibrosis in SSc is driven by aberrant activation of pro-fibrotic signaling pathways downstream of protein kinase receptors, such as the receptor for transforming growth factor beta (TGFbeta), little is known in this condition about phosphatases, which oppose signaling by kinases. We sought to explore a potential role for the low molecular weight PTP (LMPTP) in fibrotic manifestations of SSc because the LMPTP promotes growth signaling pathways in cancer cells, is highly expressed in fibroblasts and complete knockout (KO) of the LMPTP does not induce any spontaneous phenotype.
Methods: Skin biopsies and lung explants from patients with early SSc were subjected to in situ hybridization for LMPTP. Primary dermal fibroblasts (DF) were subjected to treatment with two independent LMPTP inhibitors followed by assessment of TGFbeta signaling, immunoprecipitations, transcriptomics, proteomics and phospho-proteomics analysis. TGFbeta signaling reporter assays were performed in 293 cells. Mice were subjected to the bleomycin model of skin and lung fibrosis.
Results: We found that the LMPTP is upregulated in fibrotic skin of scleroderma patients (Fig. 1). An assessment of primary DF treated with small-molecule LMPTP inhibitors showed that LMPTP promotes TGFbeta signaling (Fig. 2). Mechanistically, LMPTP promotes early TGFbeta receptor (TGFBR) signaling through the dephosphorylation of a novel substrate. Global deletion of LMPTP in mice significantly attenuated the severity of skin and lung fibrosis in the bleomycin-induced model (Fig. 3). Similar results were obtained when mice were treated with an orally bioavailable LMPTP inhibitor.
Conclusion: Taken together, these findings indicate a critical role for LMPTP in development of fibrosis through a novel mechanism of regulation of early TGFbeta signaling. As LMPTP KO mice are healthy and viable and LMPTP might be tractable for small molecule discovery, our study suggests that LMPTP inhibition is a potentially safe therapeutic strategy for preventing or treating fibrosis in scleroderma.
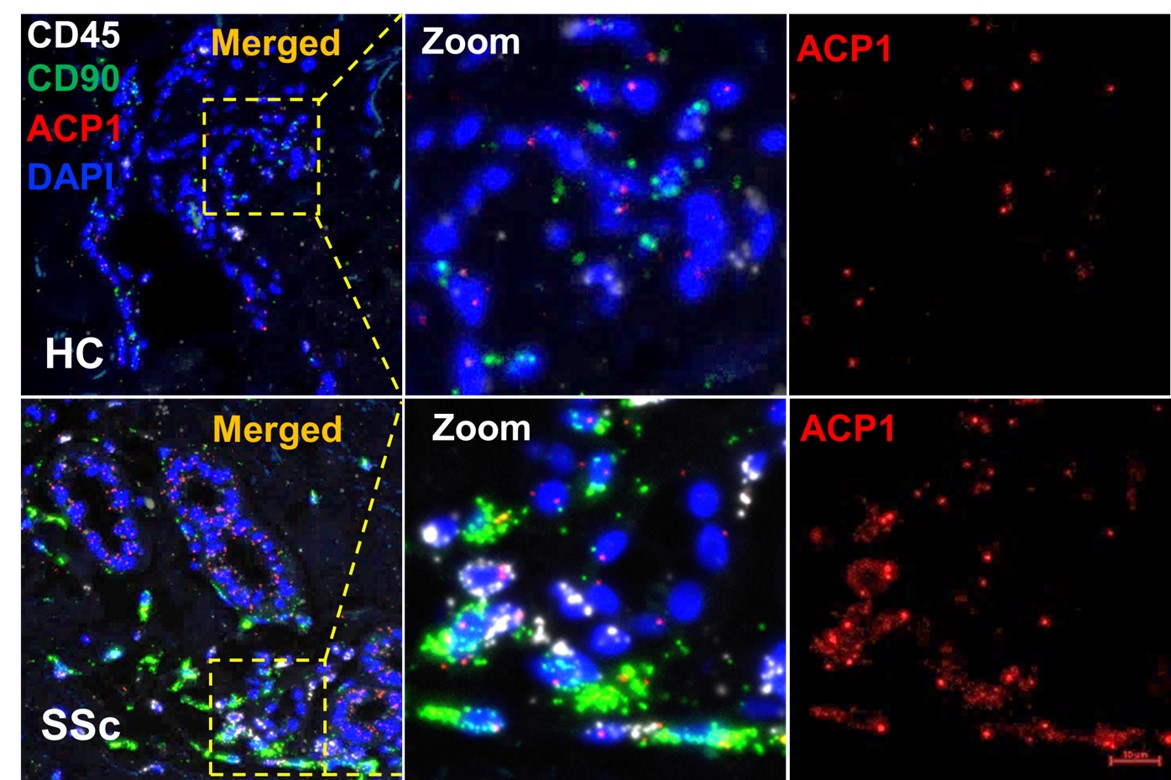 Figure 1. LMPTP expression is significantly upregulated in affected skin of SSc patients. LMPTP mRNA was quantified by RNAScope in situ hybridization using LMPTP (ACP1, red) , CD90 (fibroblast, green), CD45 (leucocytes, white) probes in both healthy (HC, n=4) and SSc patients (SSc, n=4) skin biopsies.
Figure 1. LMPTP expression is significantly upregulated in affected skin of SSc patients. LMPTP mRNA was quantified by RNAScope in situ hybridization using LMPTP (ACP1, red) , CD90 (fibroblast, green), CD45 (leucocytes, white) probes in both healthy (HC, n=4) and SSc patients (SSc, n=4) skin biopsies.
.jpg) Figure 2. LMPTP inhibition reduces pro-fibrotic gene expression and dampens SMAD-dependent TGF-β signaling. A. qPCR analysis of COL1A1, COL1A2, and ACTA2 expression in human dermal fibroblasts treated with 10 µM Compound 23, stimulated with 10 ng TGF-beta1 for 24h. Error bars represent mean ± SEM normalized to control group. Statistical significance was determined using paired t test. **P < 0.01, ****P < 0.0001. B. SMAD-dependent luciferase reporter assay assessing SMAD3 transcriptional activity after 24-hour stimulation of 10 ng/mL TGF-β1 in 293T cells, with or without 10 μM Compound 23. Luciferase activity was normalized to Renilla. Error bars represent Mean ± SEM and representative at least three different experiments in triplicate. Statistical significance was assessed using One-way ANOVA test, **P < 0.01, ****P < 0.0001.
Figure 2. LMPTP inhibition reduces pro-fibrotic gene expression and dampens SMAD-dependent TGF-β signaling. A. qPCR analysis of COL1A1, COL1A2, and ACTA2 expression in human dermal fibroblasts treated with 10 µM Compound 23, stimulated with 10 ng TGF-beta1 for 24h. Error bars represent mean ± SEM normalized to control group. Statistical significance was determined using paired t test. **P < 0.01, ****P < 0.0001. B. SMAD-dependent luciferase reporter assay assessing SMAD3 transcriptional activity after 24-hour stimulation of 10 ng/mL TGF-β1 in 293T cells, with or without 10 μM Compound 23. Luciferase activity was normalized to Renilla. Error bars represent Mean ± SEM and representative at least three different experiments in triplicate. Statistical significance was assessed using One-way ANOVA test, **P < 0.01, ****P < 0.0001.
.jpg) Figure 3. LMPTP promotes bleomycin-induced fibrosis in vivo.
Figure 3. LMPTP promotes bleomycin-induced fibrosis in vivo.
A. Schematic overview of tamoxifen-inducible ACP1 knockout mouse model and bleomycin-induced fibrosis protocol. B. Masson’s trichrome and collagen-specific fluorescent staining (F-CHP) of skin and lung from wild-type and ACP1 KO mice after bleomycin treatment (Cre+, n=26; Cre−, n=20). Blue dots represent male samples and red dots represent female samples. C. Quantification of dermal thickness, collagen deposition, and fluorescence intensity in skin and lung. Data are shown as mean ± SEM. Statistical significance was determined using the Mann-Whitney test. *P < 0.05, **P < 0.01, ****P < 0.0001.
To cite this abstract in AMA style:
Zhan Y, Sanders C, Diaz M, Miao J, Kettenbach A, Zhang Z, Wolters P, Boin F, Stanford S, Bottini N. LMPTP Drives Fibrosis in Systemic Sclerosis via TGF-beta Signaling Activation [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/lmptp-drives-fibrosis-in-systemic-sclerosis-via-tgf-beta-signaling-activation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lmptp-drives-fibrosis-in-systemic-sclerosis-via-tgf-beta-signaling-activation/
