Session Information
Date: Monday, October 27, 2025
Title: (0934–0954) Systemic Lupus Erythematosus – Animal Models Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Cognitive impairment is a frequent manifestation of neuropsychiatric systemic lupus erythematosus, present in up to 80% of patients and leading to a diminished quality of life.
Methods: We used a model of lupus-like cognitive impairment that is initiated when antibodies that cross-react with DNA and excitatory neuronal receptors penetrate the hippocampus, causing immediate, self-limited, excitotoxic death of hippocampal neurons, which is then followed by a significant loss of dendritic complexity in surviving neurons and impairment in spatial memory.
Results: This injury creates a maladaptive equilibrium that is sustained in mice for at least 1 year. We identified a feedforward loop of microglial activation and microglia-dependent synapse elimination dependent on neuronal secretion of high mobility group box 1 protein (HMGB1) which binds the receptor for advanced glycation end products (RAGE) and leads to microglial secretion of C1q, upregulation of interleukin-10 with consequent downregulation of leukocyte-associated immunoglobulin-like receptor 1 (LAIR-1), an inhibitory receptor for C1q. While we previously showed that treatment with a centrally acting angiotensin-converting enzyme inhibitor restored a healthy equilibrium, microglial quiescence and intact spatial memory, we now show that a centrally acting angiotensin receptor blocker also is effective, confirming the angiotensin pathway as a mediator of neuronal damage.
Conclusion: We have identified a mechanism by which neuroinflammation can be sustained in a feedforward process despite no intrinsic, genetically determined abnormality of neurons or microglia and no continuing external triggers for inflammation. The inhibition of angiotensin II/angiotensin receptor 1 signaling is able to half the self-sustaining inflammatory cycle by lifting the suppression of LAIR-1, which enables microglia and neurons to return to a state of quiescence and a healthy homeostasis. This has the translational implication that there needs to be a direct manipulation of the cells in the CNS to modulate or abort the inflammatory process.
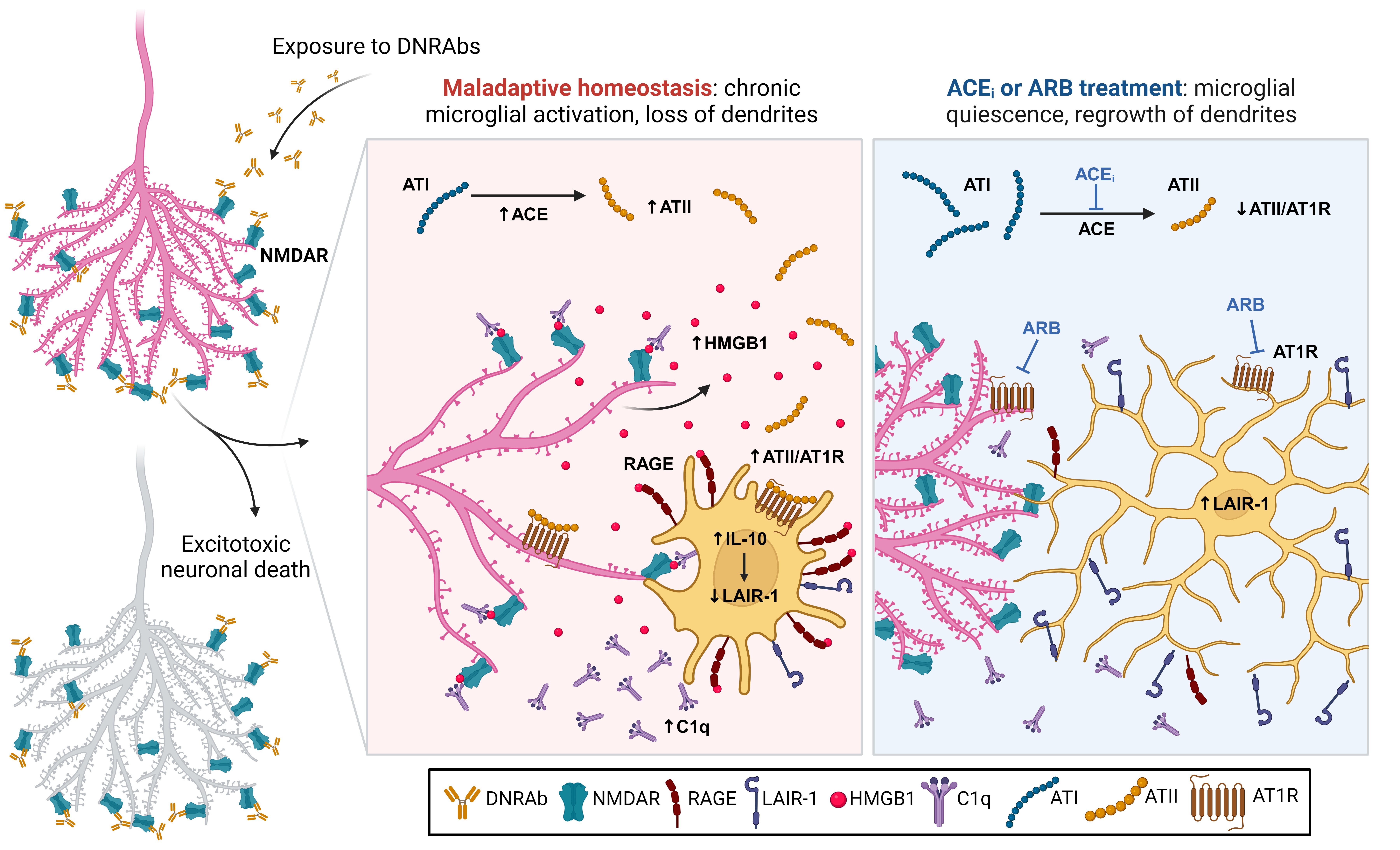 Outcomes of DNRAb-mediated neuronal damage.
Outcomes of DNRAb-mediated neuronal damage.
To cite this abstract in AMA style:
Carroll K, Mizrachi M, Simmons S, Toz B, Kowal C, Tehrani N, Zarfeshani A, Kello N, El Khoury L, Wingard J, Weissman-Tsukamoto R, Levin J, Volpe B, Diamond B. Mechanisms of anti-NMDAR antibody-mediated neuronal pathology and mitigation by angiotensin II signaling inhibition [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/mechanisms-of-anti-nmdar-antibody-mediated-neuronal-pathology-and-mitigation-by-angiotensin-ii-signaling-inhibition/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mechanisms-of-anti-nmdar-antibody-mediated-neuronal-pathology-and-mitigation-by-angiotensin-ii-signaling-inhibition/
