Session Information
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: Schnurri-3 (SHN3) is an adaptor protein and a negative regulator of osteoblast (OB) function. Its inhibition prevents bone loss and joint erosion in models of rheumatoid arthritis (RA). TNF enhances osteoclast differentiation while suppressing OB function, promoting bone loss. Previously we reported that TNF induces SHN3 transcription via p65/p50 binding at promoter position +10, while also promoting p65 binding to 2 other sites in the SHN3 promoter. However, the contribution of SHN3 to the regulation of OB differentiation under the influence of TNF is not known.
Methods: Mice were generated with constitutively active (CA) NF-κB signaling in OB lineage cells. IKK-2-CAfl/fl and IKK-2-CAfl/fl;Shn3fl/fl mice were crossed with Prx1-Cre transgenic mice. This breeding strategy produced two genotypes: IKK-2-CAPrx1_Cre mice, (CA-IKK in OB lineage cells), and Shn3fl/fl;IKK-2-CAPrx1_Cre mice (CA IKK in OB lineage-cells with deletion of SHN3). Shn3fl/fl;Prx1_Cre served as a positive control known to promote OB formation. To determine the impact of SHN3 on OB differentiation during TNF-mediated inflammation, BMSCs from Shn3WT and Shn3KO (globally deficient) mice were differentiated into OBs and treated with TNF (2 ng/mL) from days 4-18. Alkaline phosphatase staining was performed to assess OB differentiation, and RNA was harvested for bulk RNA sequencing. Data analysis was conducted using Qlucore Omics Explorer. Functional enrichment of differentially expressed genes (DEGs) in KO+TNF vs. WT+TNF cells was performed using ClueGO in Cytoscape, and selected genes were validated by qRT-PCR.
Results: Mice with constitutive activation of NF-κB in OB-lineage cells (IKK-2-CAPrx1_Cre) had reduced femoral and cortical bone volume compared with control IKK-2-CAfl/fl;Shn3fl/fl mice. Shn3 deletion in OB-lineage cells (Shn3fl/fl;IKK-2-CAPrx1_Cre) rescued this loss, and demonstrated bone mass similar to Shn3fl/fl;Prx1_Cre mice (Fig.1). In vitro, SHN3 deletion reversed TNF-induced suppression of OB differentiation, indicating that SHN3 deficiency mitigates TNF-driven suppression of OB function. RNA-seq analysis identified 211 significantly DEGs (p ≤ 0.01) between TNF-treated BMSCs from WT/KO groups. Reactome and GSEA analysis showed enrichment of type I IFN pathways in KO+TNF (Fig. 2). Notably, ISGs including Isg15, Usp18, and Ifit1/2/3 were also upregulated in KO+TNF compared with WT+TNF (Fig. 3), validated by qRT-PCR.
Conclusion: Shn3 silencing in OB-lineage cells prevents NF-κB-driven bone loss and promotes bone formation under inflammatory conditions, establishing SHN3 as an important effector of NF-κB signaling in bone. In vitro, Shn3 deletion restores OB differentiation in the presence of TNF and significantly increases the expression of ISGs, suggesting that SHN3 negatively regulates type I IFN responses in OBs during chronic inflammation. These findings compliment a report in non-inflammatory conditions showing that OB function is modulated by type I IFN and ISGs expression1. Together, our data highlight a novel axis in osteoimmunology in which SHN3 mediates TNF-induced inhibition of OB function, potentially through modulation of type I IFN signaling. Reference: 1.doi: 10.7554/eLife.59659.
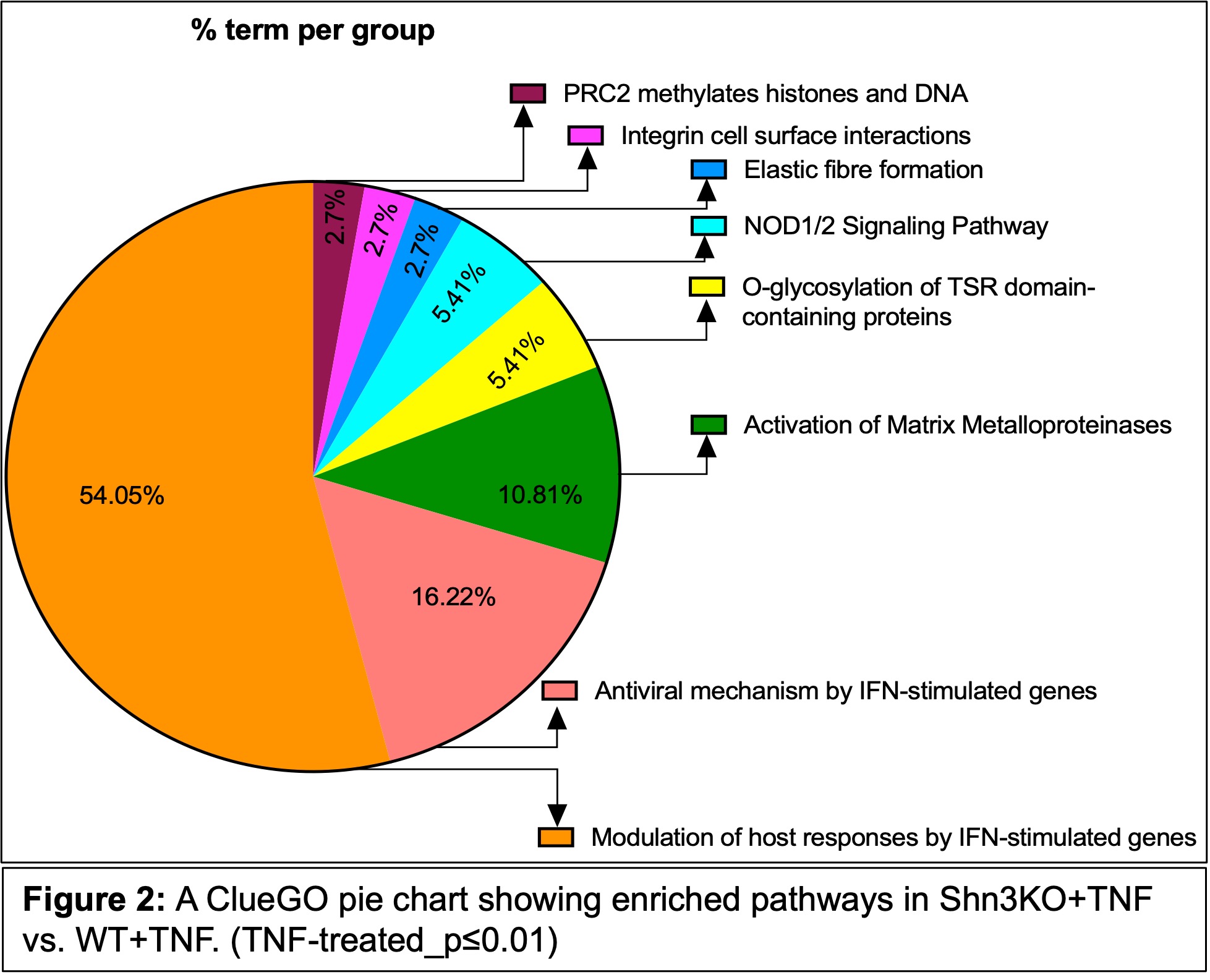 Figure 1: Deletion of Shn3 protects against NF-κB pathway-induced bone loss. MicroCT analysis was performed on 8-week-old mice as follows: IKK-2-CAfl/fl;Shn3fl/fl (control) and Prx-1Cre transgenic mice were used to generate: IKK-2-CAPrx1_Cre (CA-IKK specifically in OB lineage cells),Shn3fl/fl;;IKK-2-CAPrx1_Cre (CA-IKK-2 in OB lineage-cells with deletion of SHN3), and Shn3fl/fl;Prx1_Cre (OB lineage-cells with deletion of SHN3). Relative quantification of the femur bone parameters by microCT: (A) BV/TV (bone volume/tissue volume of trabecular bone), (B) Cs.Th (cortical bone thickness). Data are presented as mean ± SD; **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way ANOVA between groups). (n=4-6 mice).
Figure 1: Deletion of Shn3 protects against NF-κB pathway-induced bone loss. MicroCT analysis was performed on 8-week-old mice as follows: IKK-2-CAfl/fl;Shn3fl/fl (control) and Prx-1Cre transgenic mice were used to generate: IKK-2-CAPrx1_Cre (CA-IKK specifically in OB lineage cells),Shn3fl/fl;;IKK-2-CAPrx1_Cre (CA-IKK-2 in OB lineage-cells with deletion of SHN3), and Shn3fl/fl;Prx1_Cre (OB lineage-cells with deletion of SHN3). Relative quantification of the femur bone parameters by microCT: (A) BV/TV (bone volume/tissue volume of trabecular bone), (B) Cs.Th (cortical bone thickness). Data are presented as mean ± SD; **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way ANOVA between groups). (n=4-6 mice).
.jpg) Figure 2: A ClueGO pie chart showing enriched pathways in Shn3KO+TNF vs. WT+TNF. (TNF-treated_p≤0.01)
Figure 2: A ClueGO pie chart showing enriched pathways in Shn3KO+TNF vs. WT+TNF. (TNF-treated_p≤0.01)
.jpg) Figure 3: Volcano plot of RNA-seq analysis of differentially expressed genes using the mRNAs isolated from the WT and KO TNF treated groups with significance (p≤0.01).
Figure 3: Volcano plot of RNA-seq analysis of differentially expressed genes using the mRNAs isolated from the WT and KO TNF treated groups with significance (p≤0.01).
To cite this abstract in AMA style:
Kushwaha P, Tai A, Manning C, Yang Y, Shim J, Gravallese E. Silencing of Schnurri-3 protects from TNF-induced bone loss and is accompanied by upregulation of type I Interferon-Stimulated Genes (ISGs) in osteoblasts. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/silencing-of-schnurri-3-protects-from-tnf-induced-bone-loss-and-is-accompanied-by-upregulation-of-type-i-interferon-stimulated-genes-isgs-in-osteoblasts/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/silencing-of-schnurri-3-protects-from-tnf-induced-bone-loss-and-is-accompanied-by-upregulation-of-type-i-interferon-stimulated-genes-isgs-in-osteoblasts/
