Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Abatacept is a selective T-cell co-stimulation modulator approved for use in JIA. Efficacy and safety of abatacept in patients (pts) with JIA have been demonstrated previously in 2 phase 3 trials1,2 and earlier results from this phase 4 Abatacept JIA Registry (NCT01357668).3 The objective of this analysis was to provide data from a real-world setting for longitudinal safety and effectiveness of intravenous (IV) and subcutaneous (SC) abatacept in pts with JIA.
Methods: By protocol, clinical sites in the Pediatric Rheumatology Collaborative Study Group and Pediatric Rheumatology International Trial Organization enrolled pts meeting the ILAR criteria for 1 of the categories of JIA and currently taking or starting IV or SC abatacept. Planned duration of follow-up (FU) is 10 years; data were collected up to March 31, 2024. Effectiveness was assessed at day of entry into registry (baseline [BL]), 3 and 6 months, and 1, 2, 3, 4, and 5 years. Safety data were collected at each visit.
Results: Of the 740 pts enrolled, 701 were included in the analysis (476 IV, 225 SC); 561/701 (80%) were female. At BL, median age was 13.6 years and 28 (4%) pts were aged 2–5 years; JIA disease duration was 4.5 years. Median abatacept treatment duration was 6.1 months and number of active joints was 1.0 (mean 2.7). JIA categories were systemic (2%), oligo (25%), polyarticular RF-negative (48%), polyarticular RF-positive (10%), psoriatic (5%), enthesitis-related (4%), and undifferentiated (6%). Total abatacept exposure was 1513.2 pt-years with a mean/median duration of abatacept treatment of 25.9/19.7 months. At 1-year FU, pts had low Physician Global Disease Activity, low Juvenile Arthritis Multidimensional Assessment Report scores, and improvement in the number of joints with active arthritis compared with BL (Table 1). A higher percentage of pts achieved clinically inactive disease after 1 year of FU vs BL (46% vs 32%; Table 1). This trend continued despite low numbers of pts with 4 and 5 years of FU. There were 11 serious infections reported (incidence rate [IR] 0.52/100 pt-years of FU, 95% CI: 0.26, 0.92; IR 0.73/100 pt-years on treatment, 95% CI: 0.36, 1.30). There were 17 autoimmune events (IR 0.80/100 pt-years of FU, 95% CI: 0.46, 1.28; IR 1.12/100 pt-years on treatment, 95% CI: 0.65, 1.80). There were 2 adverse event malignancies (n=1 each: medulloblastoma, T-cell lymphoma). No cases of tuberculosis were reported. There was 1 unrelated death (pre-existing cardiac problems).
Conclusion: In this real-world JIA cohort, abatacept was safe and well-tolerated with no new safety risks identified. This longitudinal analysis further supports the persistent effectiveness of abatacept in pts with JIA.References: 1. Brunner HI, et al. Arthritis Rheumatol 2018;70:1144-1154.2. Ruperto N, et al. Lancet 2008;372:383-391.3. Lovell DJ, et al. Rheumatology 2024;63:S1195-S1026.Medical writing: Alexus Shirk, PhD (Caudex, an IPG Health Company), funded by Bristol Myers Squibb.
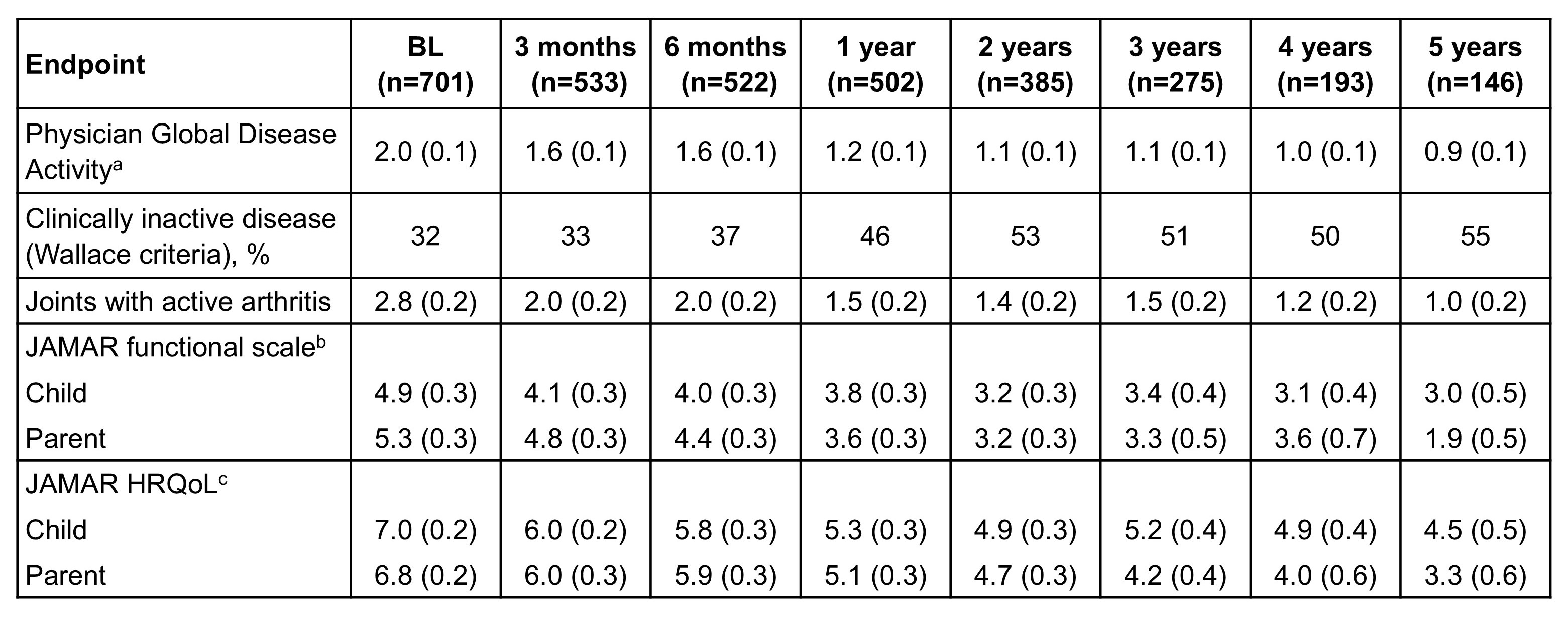 Table 1. Assessment of disease activity and impact. Data are mean (SE), unless otherwise indicated. a: Visual analog scale 0–10; 0=inactive; b: Range 0–15, 0=no functional limitation; c: Range 0–15, 0=best possible HRQoL. HRQoL, health-related quality of life; JAMAR, Juvenile Arthritis Multidimensional Assessment Report; SE, standard error.
Table 1. Assessment of disease activity and impact. Data are mean (SE), unless otherwise indicated. a: Visual analog scale 0–10; 0=inactive; b: Range 0–15, 0=no functional limitation; c: Range 0–15, 0=best possible HRQoL. HRQoL, health-related quality of life; JAMAR, Juvenile Arthritis Multidimensional Assessment Report; SE, standard error.
To cite this abstract in AMA style:
Lovell D, Ruperto N, Huggins J, Alexeeva E, Correll C, Bohnsack J, Tarvin S, Simonini G, Griffin T, Zeft A, Horneff G, Quartier P, Orban I, Walters H, Stanevica V, Patel J, Huber A, Rosenkranz M, Kingsbury D, Scuccimarri R, Vega Cornejo G, Swart J, Carroll R, Brunner H, Sherrard T, Pallotti C, Malattia C, Martini A. Longitudinal Effectiveness of Abatacept in JIA: Results From an Ongoing JIA Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/longitudinal-effectiveness-of-abatacept-in-jia-results-from-an-ongoing-jia-registry-2/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-effectiveness-of-abatacept-in-jia-results-from-an-ongoing-jia-registry-2/
