Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Juvenile Idiopathic Arthritis (JIA) is the most common pediatric rheumatic disease. Tumor necrosis factor inhibitors (TNFi) are key treatments in non-systemic JIA (sJIA), but patients diagnosed ≤2 years old have been underrepresented in studies thus far. Despite widespread clinical use, limited data exists on TNFi efficacy in this cohort, often leading to treatment delays. Early disease control is critical to optimize long-term outcomes and prevent damage. This study aimed to (1) describe demographics of children diagnosed with non-sJIA 0-23 mo and (2) evaluate TNFi effectiveness in improving disease activity in these patients using data from the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry, a national multicenter observational database.
Methods: Demographic and disease characteristics were described for all patients diagnosed with non-sJIA 0-23 mo from 2015-2023 in the CARRA Registry using descriptive statistics. Efficacy was analyzed in patients enrolled within 6 months of diagnosis with ≥2 follow-up visits at 0-3, 4-8, 10-14, or 16-20 months using total active joint count, clinical Juvenile Arthritis Disease Activity Score (cJADAS-10), and Patient-Reported Outcomes Measurement Information System (PROMIS) T-scores. Bivariate analysis comparing the subcohort to the entire cohort was performed using Mann Whitney U and Chi-Square tests with a significance of 0.05. Effects size was reported based on the type of statistical test used.
Results: 949 children were diagnosed with non-sJIA 0-23 mo in the CARRA registry; 29.3% were enrolled within 6 months of diagnosis and had ≥2 follow-ups. Most common subtypes at enrollment were oligoarticular JIA (54.0%) then Rheumatoid Factor (RF)-negative polyarticular JIA (35.1%). Early enrollees had higher baseline disease activity based on active joint counts (2 vs 0), physician global assessments (PGA) (2 vs 0), childhood health assessment questionnaires (CHAQ) (0.375 vs 0.125), and cJADAS10 (8 vs 3) (p< 0.001). They also had higher ANA positivity rates (76.9% vs. 71.3%, p=0.02) and lower uveitis rates (13.4% vs 32.0%, p< 0.001) (Table 1). At baseline, the TNFi ≤2 patients had higher mean active joint counts (4 vs 3 vs 2), cJADAS10 scores (14.5 vs 10 vs 7), and lower PROMIS scores (30.5 vs 25.5 vs 39) compared to the TNFi >2 and never-TNFi groups respectively. Improvements were seen through 16-20 mo with similar joint counts (0 vs 0 vs 0), cJADAS10 (0.75 vs 1 vs 0), and PROMIS (56 vs 56 vs 45) across all 3 groups (Fig 1).
Conclusion: Patients enrolled closer to diagnosis had higher baseline disease activity, reinforcing that they represent an “enriched” cohort and more severe phenotype. Despite this, patients who started TNFi ≤2 demonstrated significant and sustained improvements in all disease activity measures by 12 months, ultimately reaching similar values as those who started TNFi >2 and did not use TNFi. This suggests that early initiation of TNFi therapy has a significant impact on disease course as it essentially corrected for the initial dramatically higher values in disease activity. These Improvements across clinical and functional measures strongly support the efficacy and early initiation of TNFi in the youngest children with non-sJIA.
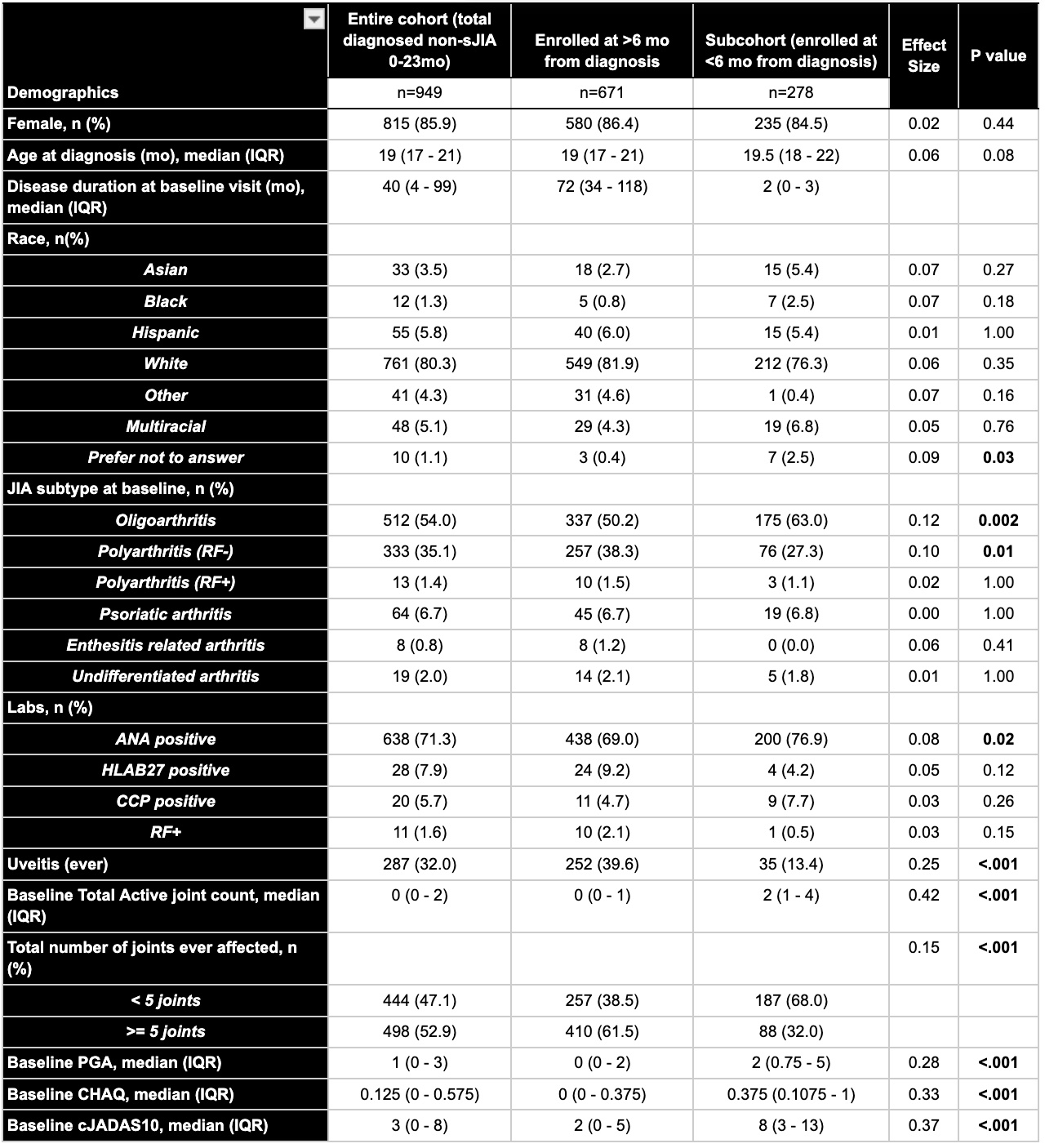 Table 1: Demographics and Clinical Disease Characteristics For All Patients Diagnosed With Non-sJIA 0-23 Months and Those Enrolled in CARRA Registry More and Less Than 6 Months From Diagnosis
Table 1: Demographics and Clinical Disease Characteristics For All Patients Diagnosed With Non-sJIA 0-23 Months and Those Enrolled in CARRA Registry More and Less Than 6 Months From Diagnosis
.jpg) Figure 1: Disease Activity Over Time in Those That Started TNFi < 2 Years Old, TNFi ≥ 2 Years Old, and Never Used TNFi
Figure 1: Disease Activity Over Time in Those That Started TNFi < 2 Years Old, TNFi ≥ 2 Years Old, and Never Used TNFi
To cite this abstract in AMA style:
Gulla C, Lozy T, Janow G. Efficacy of Tumor Necrosis Factor Inhibitors in Children Diagnosed With Non-Systemic Juvenile Idiopathic Arthritis ≤ 2 Years Old Using The Childhood Arthritis and Rheumatology Research Alliance Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-tumor-necrosis-factor-inhibitors-in-children-diagnosed-with-non-systemic-juvenile-idiopathic-arthritis-%e2%89%a4-2-years-old-using-the-childhood-arthritis-and-rheumatology-research-allianc/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-tumor-necrosis-factor-inhibitors-in-children-diagnosed-with-non-systemic-juvenile-idiopathic-arthritis-%e2%89%a4-2-years-old-using-the-childhood-arthritis-and-rheumatology-research-allianc/
