Session Information
Date: Sunday, October 26, 2025
Title: (0357–0386) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: While glucocorticoids (GCs) are commonly used to treat a range of inflammatory diseases, the burden of toxicities associated with these medications is significant. The Glucocorticoid Toxicity Index is a clinician-reported outcome measure that quantifies change in steroid toxicity over time. A robust complementary patient-reported outcome measure (PRO) is needed to capture the patient perspective of steroid treatment. The purpose of this study was to develop and assess the content validity of a PRO, the Patient Reported Outcome of Steroid Experience (PRO-SE), to evaluate GC toxicity. The PRO-SE was developed following established PRO guidance.
Methods: The draft PRO-SE was developed from a pool of concepts derived from one-on-one virtual patient interviews. Individual hybrid concept elicitation/cognitive interviews were conducted with patients with a range of conditions for which GCs are prescribed. Patients were recruited through patient advocacy organizations and interviewed via telephone or video conference. During the concept elicitation portion of the interview, participants shared symptoms associated with their illness as well as toxicities associated with GC medication. Participants then completed the PRO-SE and provided feedback on their comprehension of the instrument as well as the clarity and relevance of the items.
Results: Interviews were completed with 20 participants (n=3 IgG4-related disease; n=4 vasculitis; n=1 bullous pemphigoid; n=3 systemic lupus erythematosus; n=3 chronic inflammatory demyelinating polyradiculoneuropathy; n=6 myasthenia gravis). Toxicities commonly experienced by participants included difficulty sleeping/insomnia (n=10, 50%), swelling in the face (“moon face”; n=8, 40%), weight gain (n=7, 35%), difficulty with skin healing, increased appetite, and mood swings (all n=5, 25%). Participant responses to the PRO-SE are shown in Figure 1. When asked to assess the measure, all participants (n=20, 100%) provided positive feedback, describing it as “good,” “comprehensive,” “easy,” “simple,” or “clear.” Participants also demonstrated understanding of the instructions, 7-day recall period, and response options (n=20, 100%). There were no major item comprehension issues, with 95-100% of the participants clearly demonstrating understanding of each of the 16 items. Participants stated that the most important items were fatigue (n=16, 80%), sleep problems (n=15, 75%), facial swelling, and difficulty controlling appetite (both n=11, 55%; not mutually exclusive).
Conclusion: The PRO-SE demonstrated good content validity in this population. Participants found the PRO-SE comprehensive, easy to complete and relevant to their experience with GC toxicity. Further research should be conducted to assess psychometric validity and determine a scoring algorithm for this new instrument. The PRO-SE is a potentially useful tool for assessing patients’ experience of GC toxicity in clinical practice and may serve as either a stand-alone instrument or as a complement to clinician ratings of steroid toxicity, for example, the Glucocorticoid Toxicity Index.
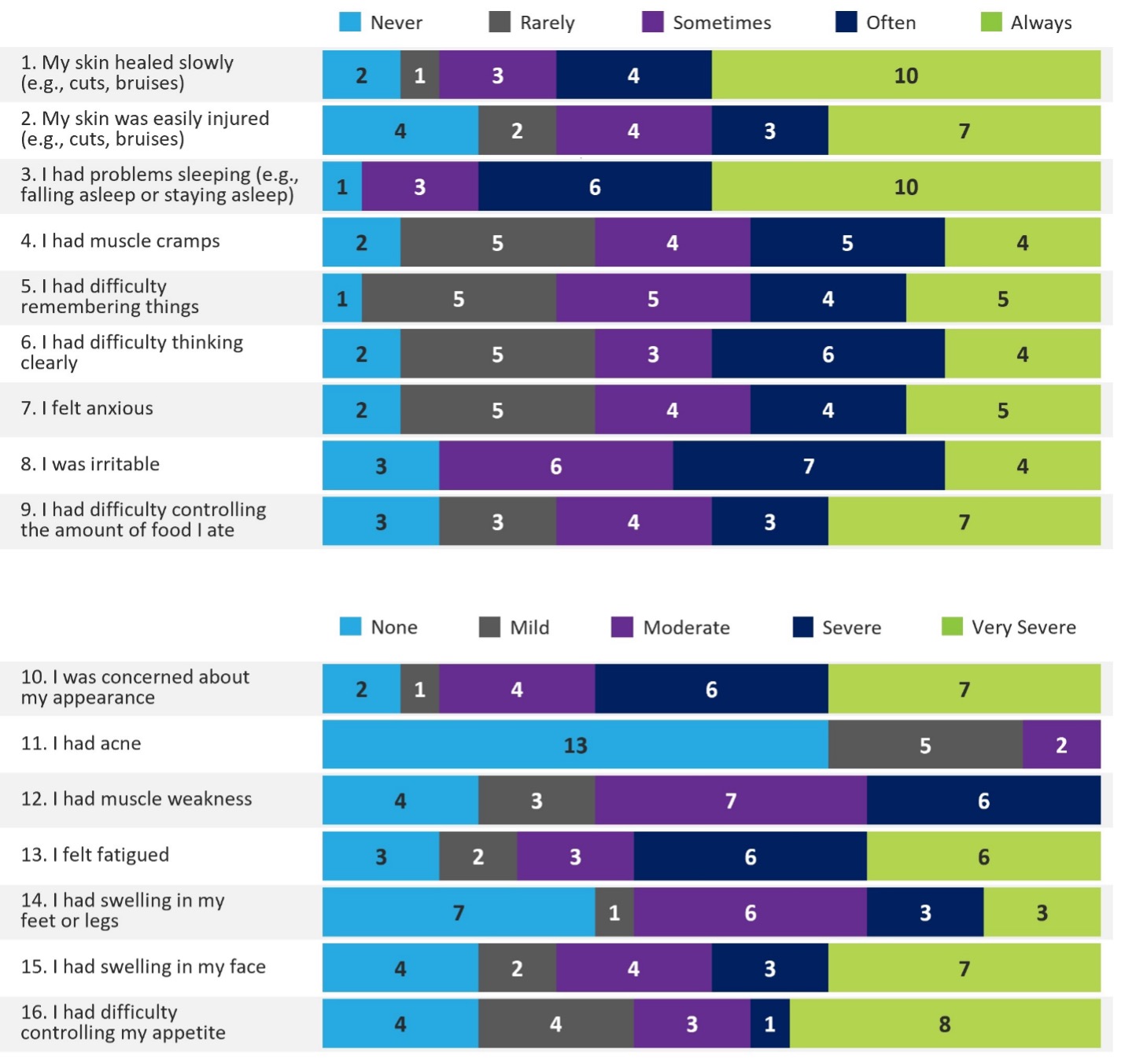 Participant Responses to the PRO-SE
Participant Responses to the PRO-SE
To cite this abstract in AMA style:
Howell T, Skalicky a, Matza L, Stone J, Stone M, Rao V, Phillips G. Assessing Content Validity of a New Questionnaire Evaluating Glucocorticoid Toxicity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/assessing-content-validity-of-a-new-questionnaire-evaluating-glucocorticoid-toxicity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-content-validity-of-a-new-questionnaire-evaluating-glucocorticoid-toxicity/
