Session Information
Date: Sunday, October 26, 2025
Title: (0280–0305) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Idiopathic inflammatory myopathies (IIM) present with diverse clinical features and disease courses. The myositis autoantibody line blot immunoassay (LIA) has advanced IIM diagnostics and is widely adopted across multiple specialties. We explored its predictive value in real-world, broader clinical settings.
Methods: A retrospective observational study of consecutive patients received the myositis autoantibody LIA testing in the National Taiwan University Hospital (NTUH) System from April 2021 to March 2023. All patients received the LIA testing via EUROLINE Autoimmune Inflammatory Myopathies 16 Ag kit. Patient demographics, including the presenting symptoms and IIM diagnosis subtypes, LIA details and the associated diagnostic work-up results were extracted from the electronic medical records (EMR). Two adult rheumatologists (TY and PH) were involved for the clinical review.Based on the presence of IIM and the LIA results, we categorized the autoantibody status of the each patients into: true-positive (TP, IIM+Autoantibody+), false-positive (FP, IIM-Autoantibody+), false-negative (FN, IIM+Antoantibody-), true-negative (TN, IIM-Antoantibody-) and calculated the clinical predictive values. We further determined the LIA testing indications for each patients into: suspected IIM (presented with manifestations across multiple clinical domains), single clinical domain (pulmonary, muscular, joint, skin, cardiovascular) and autoimmune work-ups. We then analyzed autoantibody positive predictive value (PPV) based on the LIA testing indication categories. All analysis were performed using R software.
Results: A total of 677 patients were included. One hundred sixty-two patients were diagnosed with IIM and the most common subtypes ere dermatomyositis (73, 45.1%), anti-synthetase syndrome (45, 27.8%) and polymyositis (24, 14.8%)(Table 1). LIA demonstrated overall sensitivity 80.2% and specificity 66.4%. Suspected IIM exhibited the highest PPV (95.9%) and positive likelihood ratio than any entities from single clinical domain or autoimmune work-ups (Figure 1)Among patients diagnosed with IIM, 19.7% were LIA negative while MDA5 (11.1%), multiple MSA (9.9%) and TIF1r (9.3%) were the most commonly positive autoantibodies. While PPV of most autoantibodies were high for suspected IIM even at low positivity (Figure 2A), autoantibodies with higher PPV for pulmonary manifestations (Figure 2B) were multiple coexisting anti-aminoacyl-tRNA synthetase antibodies, TIF1r and MDA5. Autoantibody PPV was low for cases tested for joint, skin manifestations and autoimmune work-ups (Figure 2C).
Conclusion: Pre-test clinical assessment is valuable under wider clinical applications as the diagnostic accuracies increase with pre-test probability. Autoantibody positive predictive values vary with specific autoantibodies and clinical Indications. Further studies are necessary to elucidate whether myositis autoantibody positivity in cases tested for single clinical domain manifestations, in particular the pulmonary manifestations, would predict and/or associate with disease course and treatment response.
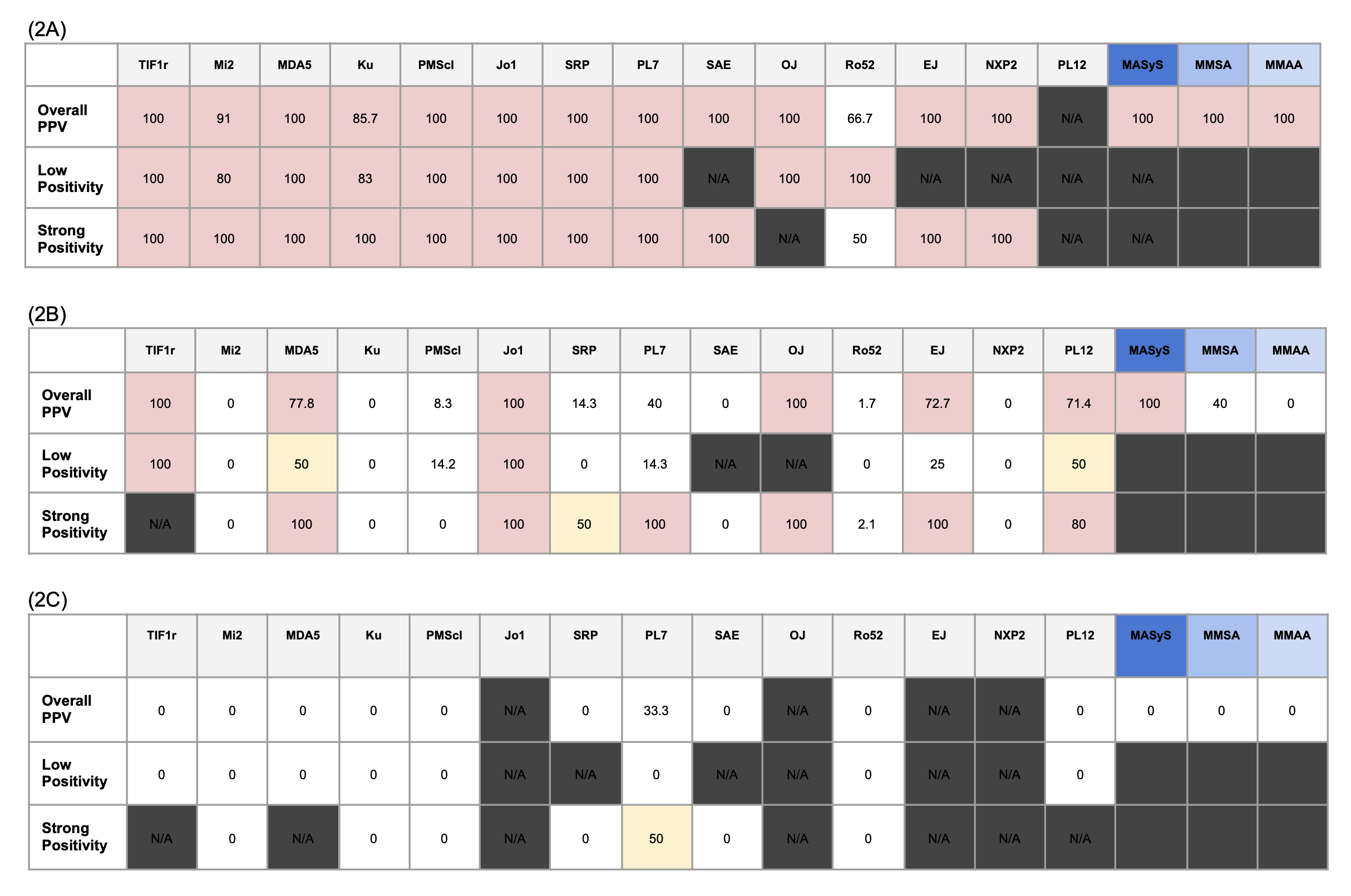 Table 1. Characteristics of the included cases.
Table 1. Characteristics of the included cases.
.jpg) Figure 1. Myositis Autoantibody Line Blot Immunoassay (LIA) Clinical Performances, Stratified by Testing Indications.
Figure 1. Myositis Autoantibody Line Blot Immunoassay (LIA) Clinical Performances, Stratified by Testing Indications.
Heatmap demonstrating positive predictive value and positive likelihood ratio of myositis LIA, stratified by testing indications. Both the highest PPV and LR+ were seen in the suspected IIM category.
IIM: idiopathic inflammatory myopathy. PPV: positive predictive value. LR+: positive likelihood ratio.
.jpg) Figure 2. Myositis Autoantibody Positive Predictive Value (PPV), Stratified by LIA Testing Indications.
Figure 2. Myositis Autoantibody Positive Predictive Value (PPV), Stratified by LIA Testing Indications.
Heatmap demonstrating PPV for individual autoantibody PPV(%) among the cases with LIA tested for (2A) Suspected IIM, (2B) Pulmonary manifestations and (2C) autoimmune work-ups. Multiple autoantibody positivity was distinguished from single antibody positivity. Overall positivity PPV included PPV of both low and strong positivity.
MMSA: presence of more than one myositis specific antibody. MASyS: presence of more than one anti-aminoacyl-tRNA synthetase antibody. MMAA: presence of more than one myositis associated antibody. N/A: non-applicable.
To cite this abstract in AMA style:
lai p, LAN T, Cheng C, Lee T, Chang T, Kao J, Lin K, Lu C, Shen C, Li K, Hsieh S. Interpreting Myositis Autoantibody Line-Blot Immunoassays in Real-World Settings: Implications for Diagnostic Accuracy for Inflammatory Myopathies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/interpreting-myositis-autoantibody-line-blot-immunoassays-in-real-world-settings-implications-for-diagnostic-accuracy-for-inflammatory-myopathies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interpreting-myositis-autoantibody-line-blot-immunoassays-in-real-world-settings-implications-for-diagnostic-accuracy-for-inflammatory-myopathies/
