Session Information
Date: Sunday, October 26, 2025
Title: (0067–0097) Rheumatoid Arthritis – Etiology and Pathogenesis Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The discovery of circulating fibroblast-like synoviocyte (FLS), known as PRIME cells, in RA patients suggests that synovial FLS may enter the circulation through blood vessels and contribute to systemic inflammation. This study aimed to detect trans-endothelial migration of FLS and dissect the molecular crosstalk between FLS and endothelial cells (EC) during this process and its relevance in rheumatoid inflammation.
Methods: Human and mouse arthritic synovium were analyzed using immunofluorescence and RNA in situ hybridization (ISH) to identify trans-endothelial migrating FLS. An in vitro trans-endothelial migration model was established using RA FLS and human umbilical vein ECs (HUVEC), followed by transcriptomics and proteomic analysis. Spatial transcriptomics was performed on RA synovial biopsies. Functional experiments were conducted through gene knockdown in vitro and in vivo.
Results: We identified FLS transmigration through EC layers of capillaries in RA synovium, but not in osteoarthritis (OA), which correlated with RA disease activity. ISH confirmed the presence of intravascular FLS in human and mouse arthritic synovium (Fig. 1A, B, C, R2=0.6, P=0.0004). In vitro trans-endothelial migration assay demonstrated that RA FLS actively transmigrate across EC layers. Bulk RNA-seq of trans-endothelial migrated FLS identified increased expression of polycystin-1 (PKD1) (Fig. 2A). Integration of transcriptomics and proteomic data identified cathepsin K (CTSK) as upregulated in EC after transmigration of FLS (Fig 2B), Spatial transcriptomics studies showed that PKD1⁺ FLS are located in close proximity to CTSK⁺ ECs in RA synovium, supporting a spatially organized induction of CTSK by FLS in EC. In vitro assays confirmed that PKD1 knockdown significantly impaired FLS transmigration across EC layers while PKD1 knockdown in FLS reduced the severity of arthritis in vivo (Fig. 3A, B). CTSK knockdown in ECs exposed to FLS impaired angiogenesis while systemic administration of the CTSK inhibitor Odanacatib attenuated joint inflammation and vascular leakage in arthritic mice.
Conclusion: Our findings demonstrate that FLS undergo trans-endothelial migration in RA synovium and identify a mechanism driven by such migration and involving PKD1 and CTSK, which amplifies EC-driven inflammation and RA severity. These results might also provide mechanistic insight into the generation of PRIME cells and highlight FLS–EC spatial interactions as a potential therapeutic target in RA.
 (A) Left: trans-EC migration of RA FLS. Representative microscopy of RA (n=5) synovium subjected to RNA in situ hybridization (ISH) for Podoplanin (PDPN, yellow), Fibroblast Activating Protein (FAP, green), Platelet/Endothelial Cell Adhesion Molecule 1 (PECAM1, red), and Hoechst (blue). Scale bar: 100 µm. Right: Representative quantification of ISH intensities through a capillary (≤ 10 micron) wall and lumen in RA synovium. Data were analyzed using Zen (black edition).
(A) Left: trans-EC migration of RA FLS. Representative microscopy of RA (n=5) synovium subjected to RNA in situ hybridization (ISH) for Podoplanin (PDPN, yellow), Fibroblast Activating Protein (FAP, green), Platelet/Endothelial Cell Adhesion Molecule 1 (PECAM1, red), and Hoechst (blue). Scale bar: 100 µm. Right: Representative quantification of ISH intensities through a capillary (≤ 10 micron) wall and lumen in RA synovium. Data were analyzed using Zen (black edition).
(B) Correlation between the number of intravascular PDPN+ cells in RA biopsies (n=15) and disease activity score (DAS28). Simple linear regression. P=0.0004.
(C) Left: trans-EC migration of mouse FLS. Representative microscopy of serum transfer induced arthritis (STIA) synovium subjected to RNA ISH for Pdpn (yellow), Fap (green), and Pecam1 (red), and Hoechst (blue). Scale bar: 100 µm. Right: Representative quantification of ISH intensities through a capillary (≤ 10 micron) wall and lumen in STIA synovium. Data were analyzed using Zen (black edition).
.jpg) (A) Venn diagram from bulk RNA-seq of trans-EC migrated RA FLS (FLSe-Trans) compared to non-migrated FLS (FLSe-nonTrans) or FLS transmigrated without EC (FLSTrans), highlighting a shared set of upregulated genes, including polycystin 1 (PKD1).
(A) Venn diagram from bulk RNA-seq of trans-EC migrated RA FLS (FLSe-Trans) compared to non-migrated FLS (FLSe-nonTrans) or FLS transmigrated without EC (FLSTrans), highlighting a shared set of upregulated genes, including polycystin 1 (PKD1).
(B) Venn diagram from integrated proteomic and transcriptomic analysis of EC co-cultured with RA FLS (ECFLS) compared to EC alone, highlighting upregulated genes, including Cathepsin K (CTSK).
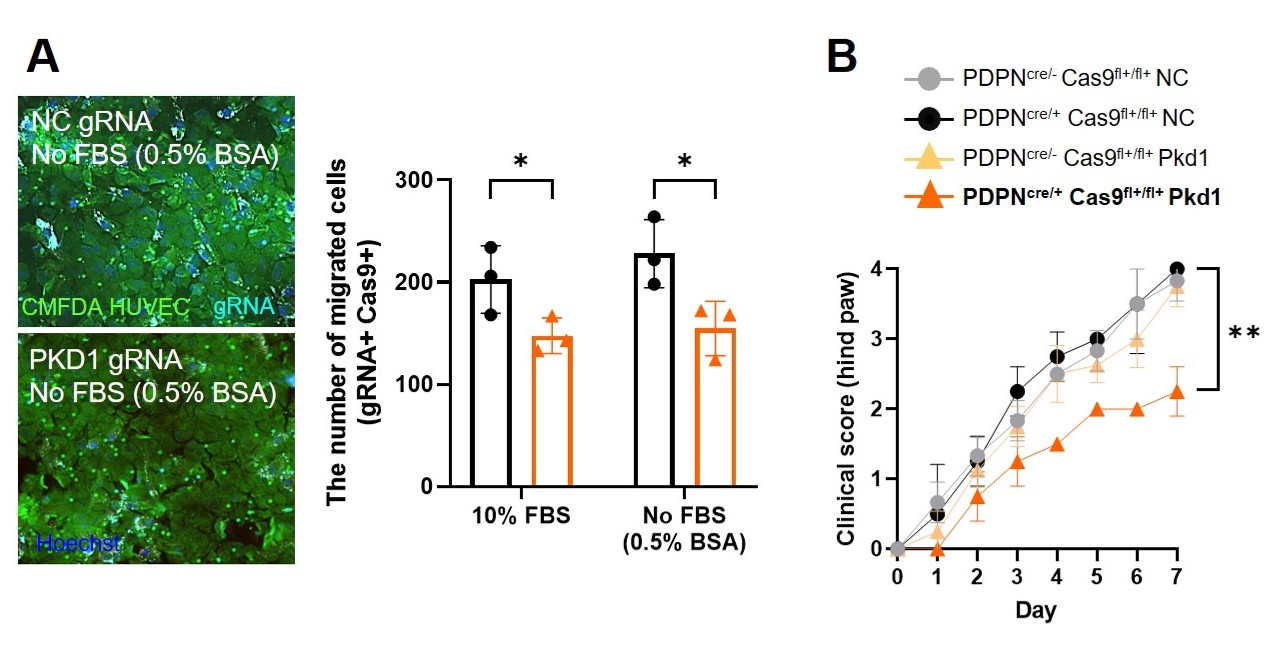
.jpg) (A) Left: representative fluorescence microscopy images from an in vitro trans-EC migration assay of RA FLS transfected with PKD1 gRNA-AF647 (cyan) compared to negative control (NC). HUVEC were loaded with 5-chloromethylfluorescein diacetate dye (CMFDA). gRNA-AF647 is cyan, , CMFDA is green, and Hoechst is blue. Right: quantification of trans-EC migrated PKD1 gRNA transfected RA FLS compared to NC gRNA under serum-containing (10% FBS) and serum-free (0.5% BSA) conditions. Each point in A (right) represents an experiment. Two-way ANOVA, Bonferroni’s multiple comparison test. *P < 0.05.
(A) Left: representative fluorescence microscopy images from an in vitro trans-EC migration assay of RA FLS transfected with PKD1 gRNA-AF647 (cyan) compared to negative control (NC). HUVEC were loaded with 5-chloromethylfluorescein diacetate dye (CMFDA). gRNA-AF647 is cyan, , CMFDA is green, and Hoechst is blue. Right: quantification of trans-EC migrated PKD1 gRNA transfected RA FLS compared to NC gRNA under serum-containing (10% FBS) and serum-free (0.5% BSA) conditions. Each point in A (right) represents an experiment. Two-way ANOVA, Bonferroni’s multiple comparison test. *P < 0.05.
(B) In vivo electroporation using tweezer electrodes and intra-articular injection of Pkd1 gRNA into joints of PDPNcre/+ Cas9 knock-in (KI) fl+/fl+ mice. Clinical arthritis scores (0 – 4) over time in PDPNcre/+ Cas9 KIfl+/fl+ and control mice following intra-articular injection of PKD1 or NC gRNA. Two-way ANOVA, Bonferroni’s multiple comparison test. **P < 0.01.
To cite this abstract in AMA style:
Kim J, Panahandeh S, Nguyen H, Mian K, Makinde H, Won K, Kettenbach A, Perlman H, Pitzalis C, Bottini N. Trans-endothelial Trafficking of Fibroblast-like Synoviocytes Amplifies Synovial Inflammation in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/trans-endothelial-trafficking-of-fibroblast-like-synoviocytes-amplifies-synovial-inflammation-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trans-endothelial-trafficking-of-fibroblast-like-synoviocytes-amplifies-synovial-inflammation-in-rheumatoid-arthritis/
