Session Information
Date: Sunday, October 26, 2025
Title: (0067–0097) Rheumatoid Arthritis – Etiology and Pathogenesis Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Genetic association data, immunohistochemistry, and functional experiments implicate protein arginine deiminase 4 (PAD4) in the pathogenesis of rheumatoid arthritis (RA). This disease is characterized by immunity against epitopes with deiminated arginine (=citrulline) originating from a multitude of intra-and extracellular proteins that are modified in this manner only in RA patients, not in healthy individuals. However, it remains uncertain how, where, and why PAD4 citrullinates these proteins in the RA patients.
Methods: To gain insights into the physical interactions of PAD4 with other cellular proteins, we identified candidate PAD4-associated proteins by mass spectrometry. PAD4 in neutrophils from RA patients and healthy controls co-immunoprecipitated with myosin-9, and 20 other proteins, many of which were also present in myosin-9 immunoprecipitates. By immunofluorescence microscopy, myosin-9 co-localized with PAD4 in RA neutrophils, as well as with myosin-9 and myosin light chain 6 in transfected 293T cells. This was confirmed by proximity ligation assays in intact neutrophils.
Results: Inhibition of the motor domain of myosin-9 by blebbistatin resulted in a more diffuse PAD4 location indicating that myosin-9 serves to transport PAD4 within the cells. However, PAD4 translocation to the nucleus involved dissociation from myosin-9. In complex with PAD4, myosin-9 was citrullinated at both N-terminal and C-terminal sites. Citrullinated peptides corresponding to these sites were recognized by IgG autoantibodies in RA patients.
Conclusion: We conclude that at least a portion of intracellular PAD4 in neutrophils interacts physically and catalytically with a myosin-9 containing macromolecular machinery involved in the cell migration and transport of organelles and membrane.
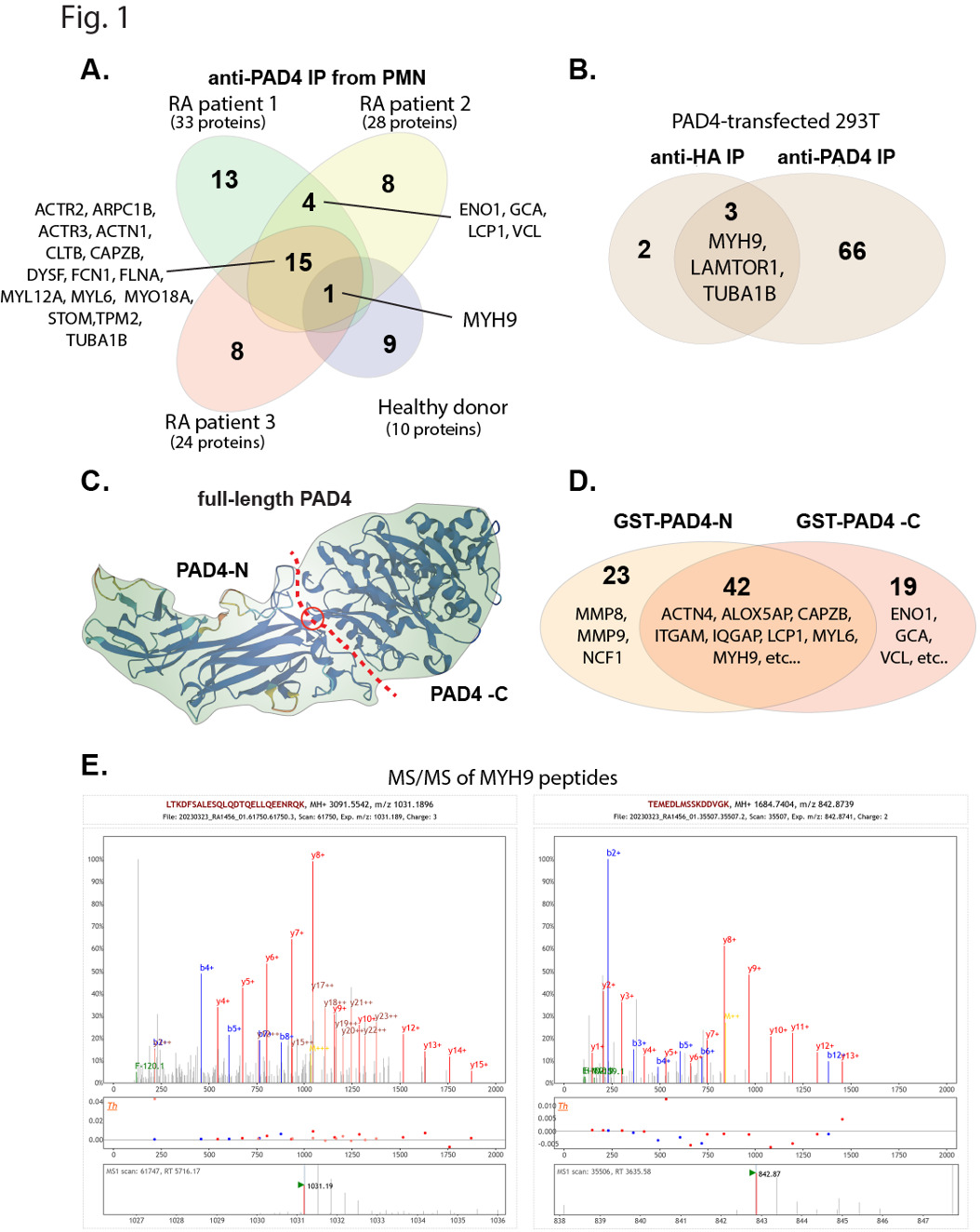 Fig. 1. Summary of mass spectrometry analyses to identify PAD4-associated proteins. A., Venn diagram of proteins present in anti-PAD4 immunoprecipitates, but not in control precipitates, from 3 RA patient and one healthy donor neutrophils. B., Venn diagram of PAD4 immunoprecipitates from transfected 293T cells. C., Illustration of the hinge (red circle) where PAD4 was cut into two halves for the construction of the two GST fusion proteins. D., Venn diagram of proteins precipitated by the N- and C-terminal halves of PAD4 fused to GST. E., Two representative mass spectra of myosin-9 peptides from the immunoprecipitates in panel A.
Fig. 1. Summary of mass spectrometry analyses to identify PAD4-associated proteins. A., Venn diagram of proteins present in anti-PAD4 immunoprecipitates, but not in control precipitates, from 3 RA patient and one healthy donor neutrophils. B., Venn diagram of PAD4 immunoprecipitates from transfected 293T cells. C., Illustration of the hinge (red circle) where PAD4 was cut into two halves for the construction of the two GST fusion proteins. D., Venn diagram of proteins precipitated by the N- and C-terminal halves of PAD4 fused to GST. E., Two representative mass spectra of myosin-9 peptides from the immunoprecipitates in panel A.
.jpg) Fig. 2. Immunofluorescence microscopy of PAD4 and putative interactors. A., RA neutrophils stained for PAD4 (green) and the indicated proteins (red): myosin-9 (top row), myosin light chain-6 (second row), grancalcin (third row), dysferlin (fourth row), stomatin (fifth row), and plastin-2 (sixth row). B. PAD4-transfected 293T cells stained for PAD4 (red) and myosin-9 (green)., C., Same cells stained for PAD4 (red) and myosin light chain-6 (green). D., Proximity-ligation assays in RA neutrophils for the pairs of PAD4 plus myosin-9, myosin light chain-6, stomatin, grancalcin, NCF1, or no second antibody, as indicated.
Fig. 2. Immunofluorescence microscopy of PAD4 and putative interactors. A., RA neutrophils stained for PAD4 (green) and the indicated proteins (red): myosin-9 (top row), myosin light chain-6 (second row), grancalcin (third row), dysferlin (fourth row), stomatin (fifth row), and plastin-2 (sixth row). B. PAD4-transfected 293T cells stained for PAD4 (red) and myosin-9 (green)., C., Same cells stained for PAD4 (red) and myosin light chain-6 (green). D., Proximity-ligation assays in RA neutrophils for the pairs of PAD4 plus myosin-9, myosin light chain-6, stomatin, grancalcin, NCF1, or no second antibody, as indicated.
.jpg) Fig. 3. Effects of myosin-9 inhibition or ionomycin on PAD4 localization. A., RA neutrophils stained for PAD4 (green) and myosin-9 (red) after a 30 min incubation at 37°C. B., Same staining of neutrophils incubated with 10 µM blebbistatin for 30 min at 37°C. C., Same for neutrophils incubated with 30 µM blebbistatin for 30 min at 37°C. D., Negative control staining with secondary antibodies alone. E., PAD4 (green) and myosin-9 (red) in neutrophils treated with 1 µM ionomycin for 30 min at 37°C. F., Magnified view of the third panel, second row, in panel E. G., PAD4 (green) and myosin-9 (red) in a neutrophil undergoing spontaneous cell death by pyroptosis. H., RA neutrophils stained for PAD4 (green) and grancalcin (red) focusing on neutrophils with nuclear PAD4. I. same in neutrophils with cytosolic PAD4. Note that grancalcin is nuclear only when PAD4 is nuclear.
Fig. 3. Effects of myosin-9 inhibition or ionomycin on PAD4 localization. A., RA neutrophils stained for PAD4 (green) and myosin-9 (red) after a 30 min incubation at 37°C. B., Same staining of neutrophils incubated with 10 µM blebbistatin for 30 min at 37°C. C., Same for neutrophils incubated with 30 µM blebbistatin for 30 min at 37°C. D., Negative control staining with secondary antibodies alone. E., PAD4 (green) and myosin-9 (red) in neutrophils treated with 1 µM ionomycin for 30 min at 37°C. F., Magnified view of the third panel, second row, in panel E. G., PAD4 (green) and myosin-9 (red) in a neutrophil undergoing spontaneous cell death by pyroptosis. H., RA neutrophils stained for PAD4 (green) and grancalcin (red) focusing on neutrophils with nuclear PAD4. I. same in neutrophils with cytosolic PAD4. Note that grancalcin is nuclear only when PAD4 is nuclear.
To cite this abstract in AMA style:
Moadab F, Shaikh F, Wang X, mustelin c, Le E, Najjar R, An J, Bays A, Mustelin T. Association of Protein Arginine Deiminase 4 with the Myosin-9 Motor Complex [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/association-of-protein-arginine-deiminase-4-with-the-myosin-9-motor-complex/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-protein-arginine-deiminase-4-with-the-myosin-9-motor-complex/
