Session Information
Date: Sunday, October 26, 2025
Title: (0067–0097) Rheumatoid Arthritis – Etiology and Pathogenesis Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Circulating concentrations of anti-malondialdehyde-acetaldehyde (MAA) antibodies distinguish patients with RA and are detectable years prior to arthritis onset. Recent data demonstrate that anti-MAA antibodies play a pathogenic role in RA by promoting osteoclastogenesis and ‘priming’ monocytes for pro-inflammatory responses. While these data suggest that anti-MAA antibodies are involved in the transition from pre-RA to clinical disease, their pathogenic role remains unknown. We evaluated whether anti-MAA antibody status is associated with differences in serum protein expression during the preclinical stage of RA development.
Methods: We used pre-diagnosis serum samples from RA cases meeting 1987 ACR criteria from the Department of Defense Serum Repository (Zaim et al., Arthritis Rheum 2025). Serum was tested for anti-MAA IgA, IgG, and IgM by ELISA, with high anti-MAA for each subtype defined as the upper quartile of all measurements. Olink Proteomics Immune Regulation, Inflammation, and Cell Regulation panels were performed on all samples (n = 197 analytes). The association of each protein (normalized expression value) with anti-MAA antibody status in longitudinally collected samples was tested via linear mixed models accounting for age, race, gender, and time to diagnosis with a subject-level random effect. P-values were adjusted for false discovery rate (FDR) and specific proteins were selected for further evaluation based on FDR-adjusted p < 0.1 and absolute log2 fold-change (FC) ≥ 0.2.
Results: A total of 122 participants with RA were included (mean age at diagnosis 37 years, 52% female), all with ≥1 pre-RA sample available (mean 1.59, Table 1). For each anti-MAA subtype, ~30% of participants had ≥1 value in the upper quartile at any time (6.6% for all 3 subtypes; Table 1). Five proteins were significantly overexpressed in the presence of high anti-MAA isotypes: 1 with IgA, 3 with IgG, and 1 with IgM (Figure 1, Table 2); most with established or suspected roles in RA pathogenesis. The largest fold-change was observed with IgM-MAA and macrophage chemotactic protein 3 (MCP3), which is highly expressed in RA synovium and promotes synovitis. The protein associated with IgA-MAA, lymphocyte activation gene-3 (LAG3), inhibits T cell activation, and high serum levels in RA are associated with autoantibody positivity and radiographic progression. IgG-MAA showed the most associations, where the largest was with transcription factor AP-1 (JUN), known to promote synovitis and bone destruction in RA. The only negative association was seen with IgG-MAA and C-type lectin domain family 4D (CLEC4D), a protein thought to have an important role in resolving inflammation.
Conclusion: Anti-MAA antibody status is associated with differences in the serum proteome during the preclinical stages of RA, particularly among mediators of inflammation and immune responses. This differential expression implicates several established pathogenic mechanisms in RA (Table 2), suggesting that anti-MAA antibodies may comprise part of a distinct pathophysiologic state leading towards progression of clinical disease during the transition from pre-RA.
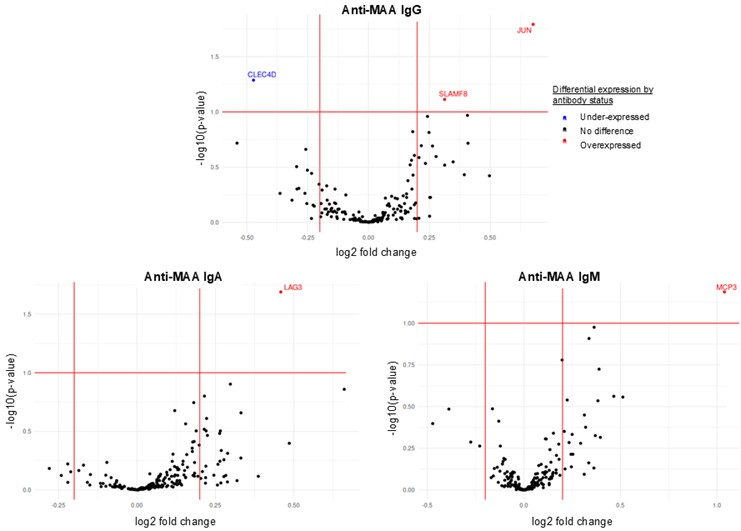 Table 1. Clinical and demographic characteristics of participants.
Table 1. Clinical and demographic characteristics of participants.
Abbreviations: malondialdehyde-acetaldehyde adduct (MAA), immunoglobulin (Ig)
Values shown as mean ± standard deviation unless specified.
.jpg) Figure 1. Volcano plots for differential protein expression based on anti-malondialdehyde adduct antibody status.
Figure 1. Volcano plots for differential protein expression based on anti-malondialdehyde adduct antibody status.
Abbreviations: malondialdehyde-acetaldehyde adduct (MAA), immunoglobulin (Ig)
.jpg) Table 2. Proteins differentially expressed based on anti-malondialdehyde antibody status in pre-RA to early RA period.
Table 2. Proteins differentially expressed based on anti-malondialdehyde antibody status in pre-RA to early RA period.
Abbreviations: immunoglobulin (Ig), lymphocyte activation gene-3 (LAG3), transcription factor AP-1 (JUN), signaling lymphocytic activation molecule family member 8 (SLAMF8), C-type lectin domain family 4 (CLEC4D), monocyte-chemotactic protein 3 (MCP3), chemokine C-C motif ligand 7 (CCL7)
Negative log2 fold change indicates inverse relationship (CLEC4D).
To cite this abstract in AMA style:
Weis E, Sayles H, Thiele G, Rachid Zaim S, Merriman T, England B, Li X, Moss L, Edison J, Feser M, Holers V, Deane K, Mikuls T, Wheeler A. Antibodies to malondialdehyde-acetaldehyde are associated with circulating inflammatory mediators during the preclinical stages of rheumatoid arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/antibodies-to-malondialdehyde-acetaldehyde-are-associated-with-circulating-inflammatory-mediators-during-the-preclinical-stages-of-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antibodies-to-malondialdehyde-acetaldehyde-are-associated-with-circulating-inflammatory-mediators-during-the-preclinical-stages-of-rheumatoid-arthritis/
