Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: IL-22 is a key cytokine involved in modulating tissue responses during inflammation and is markedly upregulated in inflammatory diseases, such as psoriasis, inflammatory bowel diseases, and rheumatoid arthritis. IL-22 binding protein (IL-22BP) inhibits IL-22 signaling by binding to it, and treatment with IL-22BP reduces the severity of cutaneous inflammation in psoriatic mice (Front Immunol. 2018;9:1418). Several studies have shown that IL-22 is critical in the pathophysiology of collagen-induced arthritis (CIA), a condition mediated by cellular and humoral immune responses (Arthritis Rheum. 2009;60:390. Arthritis Rheum. 2014;66:1492). IL-22 deficient mice develop less severe arthritis, although the effectiveness of IL-22 neutralizing antibodies in the same models varies depending on the timing of administration. While IL-22 signaling is involved in animal models of CIA, its role in autoantibody-induced arthritis remains unclear. The K/BxN serum transfer model of autoantibody-induced arthritis reflects the effector phase of arthritis and is crucial for innate immunity but not for acquired immunity. This study aimed to assess the role of IL-22 pathway in autoantibody-induced arthritis using IL-22BP-deficient mice.
Methods: C57BL/6 IL-22BP gene-deficient (-/-) mice were subjected to K/BxN serum transfer to induce arthritis, and joint inflammation was assessed histologically in a blinded manner. Murine fibroblast-like synoviocytes (mFLS) were stimulated with recombinant mouse IL-1β or IL-22. mRNA expression levels of IL-22, CXCL1 and IL-1β were analyzed in cultured cells using RT-PCR, while protein levels of IL-1β and CXCL1 were examined using ELISA. Additionally, synovial tissue expression of IL-6, IL-1β, TNFα, and CXCL1 mRNA was evaluated using RT-PCR.
Results: IL-22 mRNA expression was significantly elevated in inflamed synovial tissues of mice after K/BxN serum transfer arthritis induction (Fig. 1a). Consistent with in vivo data, mFLS can produce IL-22 mRNA after stimulation with recombinant IL-1β (Fig 1b). Disease severity was more pronounced in IL-22BP-/- mice than in wide-type (WT) controls (Fig. 1c). Histomorphometric analysis confirmed these observations, showing significantly higher histopathological scores in IL-22BP-/- mice (Fig. 1d). Consistent with these findings, the expression of pro-inflammatory genes, IL-1β, TNFα, IL-6, and CXCL1, was significantly upregulated in the inflamed synovium of IL-22BP-/- mice compared to WT controls (Fig. 2). Stimulation of mFLS with recombinant IL-22 led to increased expression of IL-1β and CXCL1 at both the mRNA and protein levels (Fig. 3a and b).
Conclusion: The IL-22/IL-22BP axis contributes to autoantibody-induced arthritis by modulating the expression of pro-inflammatory cytokines and chemokines. IL-22 directly induces mFLS to produce IL-1β and CXCL1, thereby exacerbating joint inflammation. Targeting IL-22 signaling may represent a promising therapeutic strategy for rheumatoid arthritis.
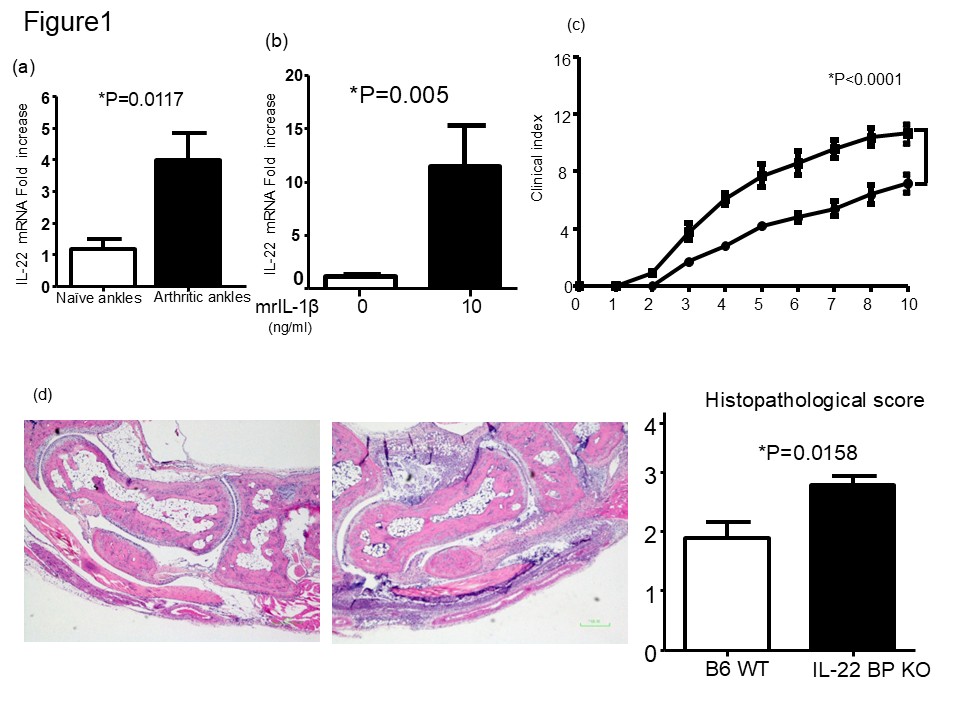 IL-22BP deficiency exacerbates K/BxN serum transfer arthritis.
IL-22BP deficiency exacerbates K/BxN serum transfer arthritis.
.jpg) Inflammatory cytokines and chemokine expression in inflamed synovial tissue during K/BxN serum transfer arthritis.
Inflammatory cytokines and chemokine expression in inflamed synovial tissue during K/BxN serum transfer arthritis.
.jpg) IL-22 induces mFLS to produce inflamatory cytokine and chemokine.
IL-22 induces mFLS to produce inflamatory cytokine and chemokine.
To cite this abstract in AMA style:
Kaieda S, Sugi S, Koga T, Kinoshita T, Hoshino T. IL-22 Contributes to Autoantibody-induced Arthritis Via Modulation of Inflammatory Cytokine and Chemokine Expression in the Inflamed Synovium of a Murine Model [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/il-22-contributes-to-autoantibody-induced-arthritis-via-modulation-of-inflammatory-cytokine-and-chemokine-expression-in-the-inflamed-synovium-of-a-murine-model/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/il-22-contributes-to-autoantibody-induced-arthritis-via-modulation-of-inflammatory-cytokine-and-chemokine-expression-in-the-inflamed-synovium-of-a-murine-model/
