Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
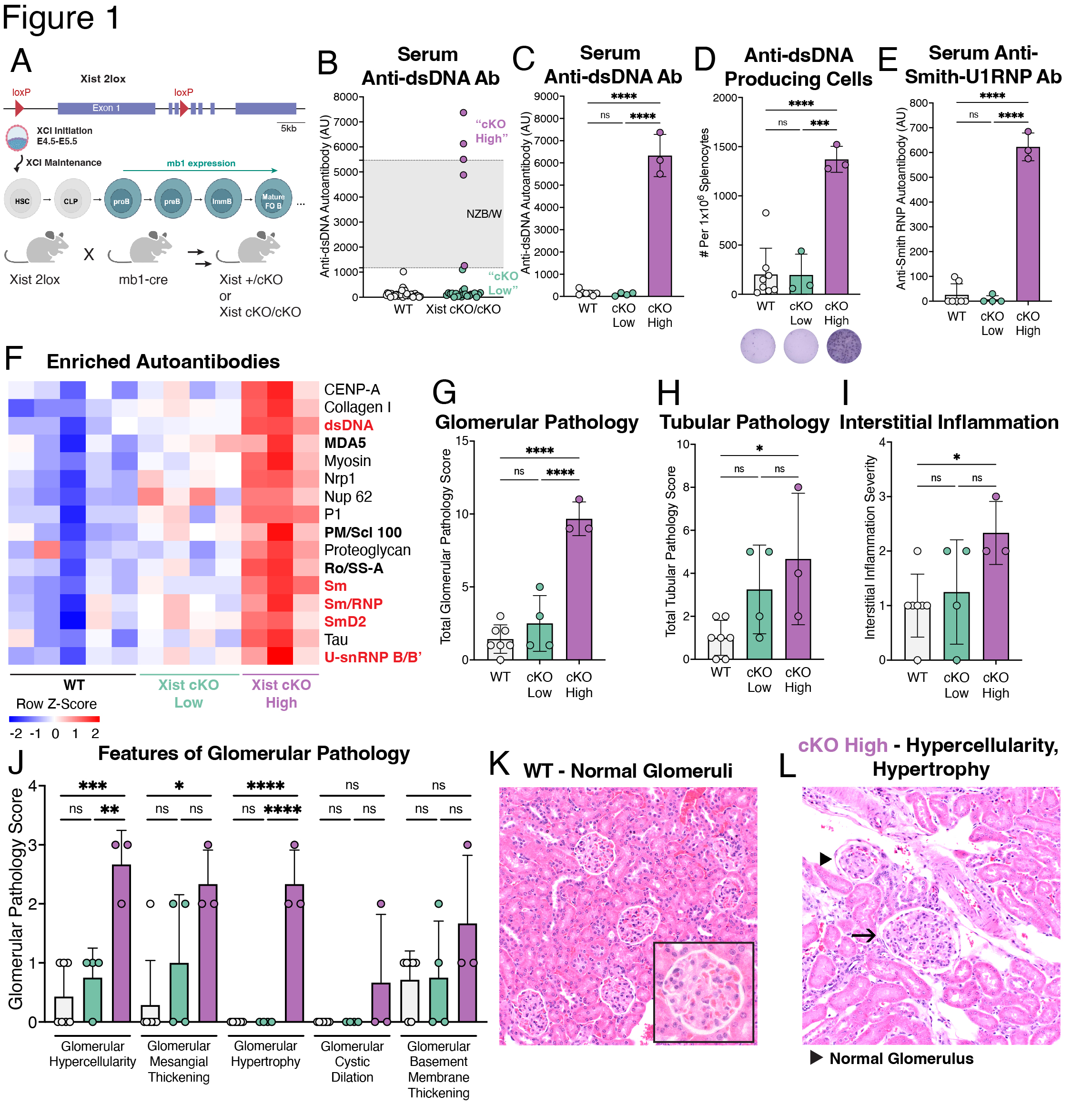
Background/Purpose: Systemic lupus erythematosus (SLE) exhibits a strong female sex bias but the mechanisms underlying this bias are not well understood. Epidemiological studies demonstrate that SLE risk increases with the number of X chromosomes carried by an individual, suggesting important genetic contributions from the X chromosome. To ensure dosage compensation of X-linked genes relative to XY males, mammals with multiple X chromosomes utilize X-chromosome inactivation (XCI), a multi-layered epigenetic mechanism that is initiated and maintained by the long non-coding RNA Xist, which is expressed from and coats the inactive X chromosome. The X chromosome is enriched for genes with immunomodulatory functions that are relevant to B cell function. B cells from female SLE patients and mouse models of SLE exhibit mislocalization of Xist RNA and aberrant expression of X-linked genes, suggesting that impairment of XCI may contribute to disease. We sought to determine whether perturbed XCI maintenance in the B cell compartment contributes to SLE pathogenesis.
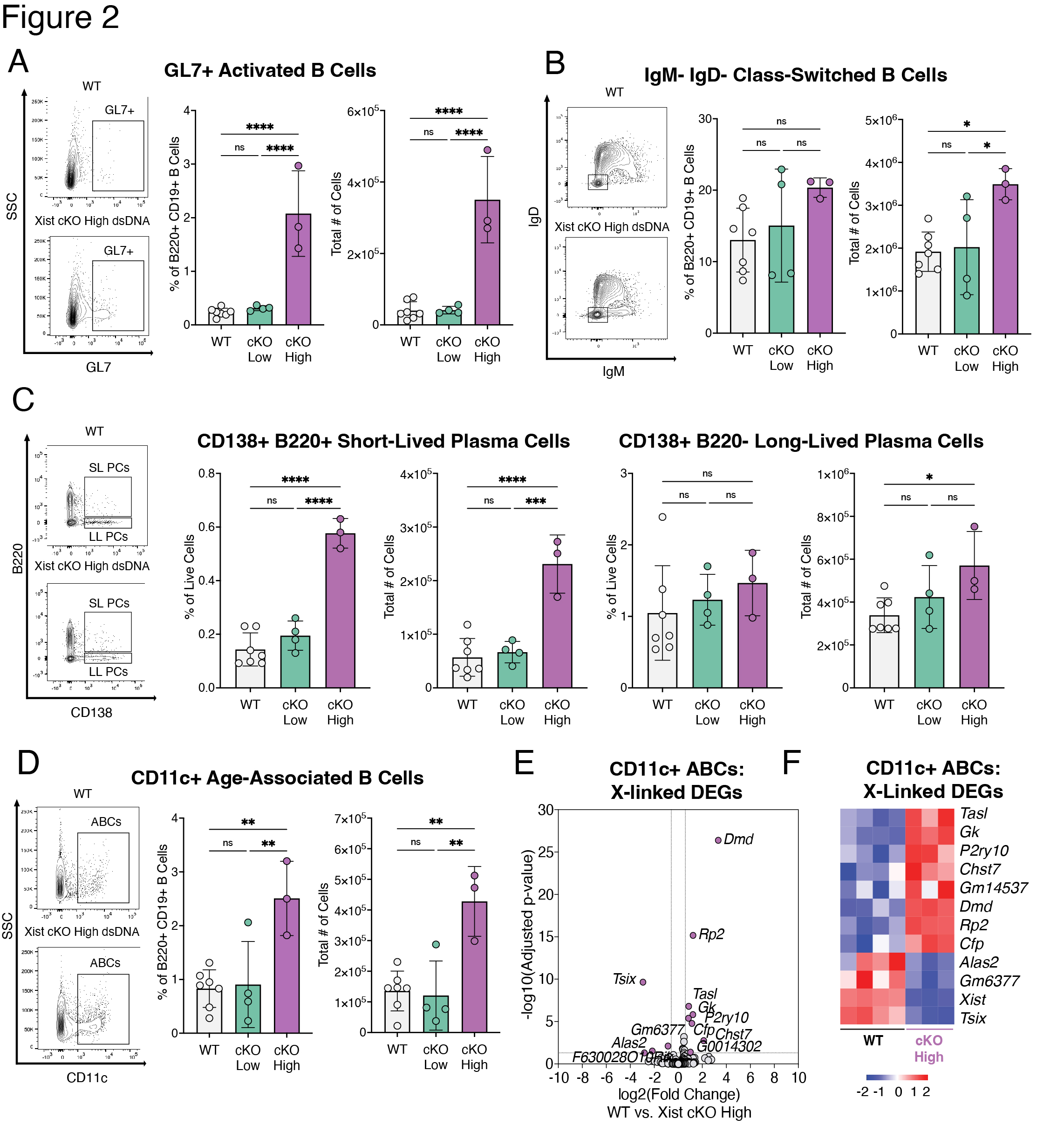
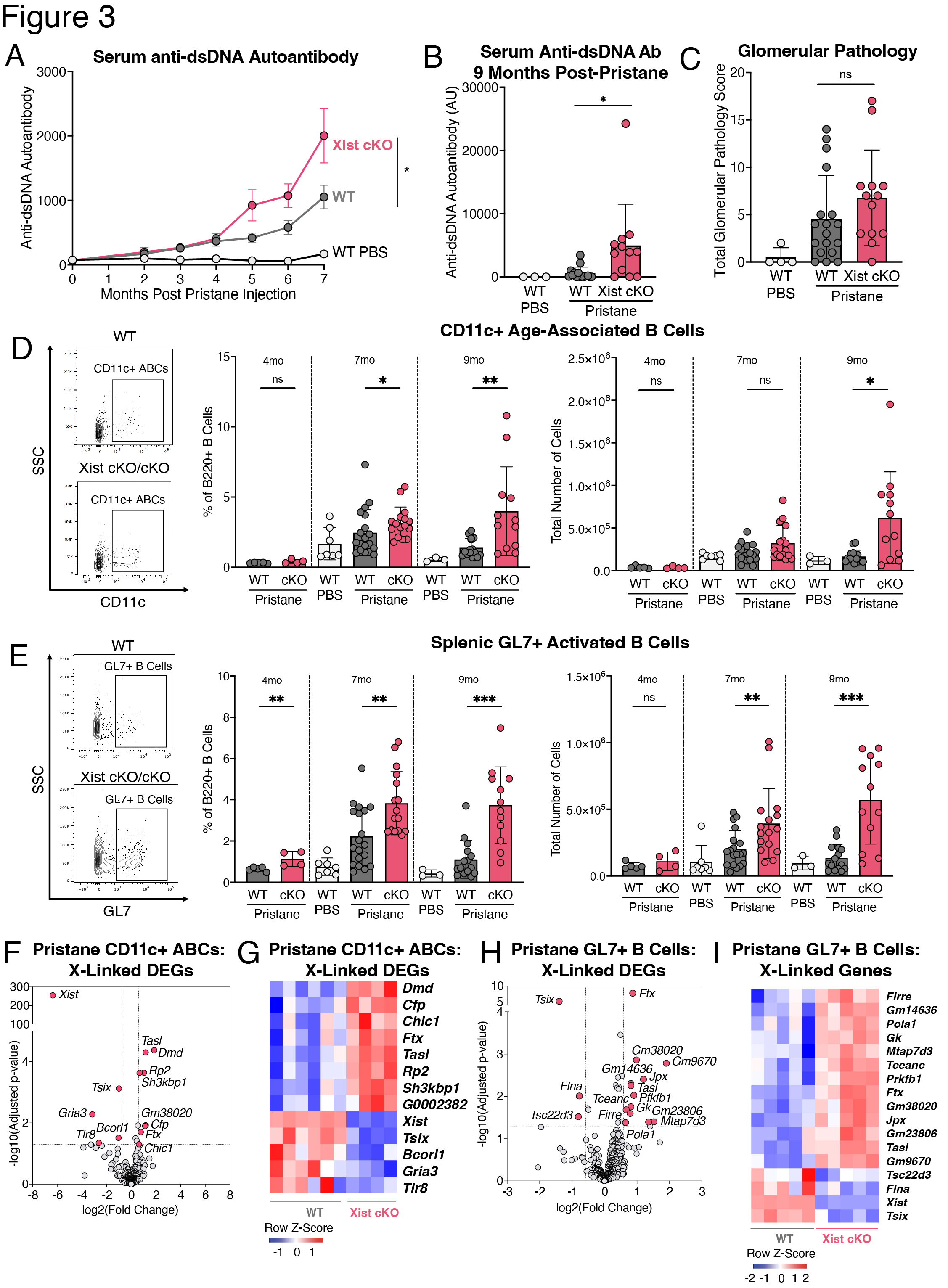
Methods: We generated female BALB/c mice with a B cell specific deletion of Xist (mb1WT/creXistfl/fl, “Xist cKO”). We measured serum levels of anti-dsDNA autoantibodies in a cohort of WT (n=109) and Xist cKO (n=66) mice. In a cohort of Xist cKO mice with high anti-dsDNA (n=3 “Xist cKO High”), Xist cKO mice with low anti-dsDNA (n=4), and WT controls (n=7) we measured sera for various autoantibodies, quantified splenic B cell subsets, and assessed kidney pathology. We also injected a cohort of WT (n=46) and Xist cKO mice (n=36) with pristane to induce SLE-like disease and measured anti-dsDNA autoantibodies, kidney pathology, and splenic B cells during disease. We performed RNAseq on activated B cells from Xist cKO mice with spontaneous and pristane-induced SLE.
Results: Some Xist cKO mice spontaneously developed elevated anti-dsDNA, anti-Smith U1-RNP, anti-MDA5, anti-PM/Scl-100, and anti-Ro/SS-A, as well as glomerular kidney pathology. These Xist cKO mice also had more activated B cell subsets including GL7+ activated B cells, class-switched B cells, age-associated B cells (ABCs), and plasma cells. Pristane-treated Xist cKO mice developed higher levels of anti-dsDNA, had slightly more severe glomerular kidney pathology, and had more activated B cell subsets compared to pristane-treated WT mice. Activated B cells from Xist cKO mice with spontaneous and pristane-induced SLE demonstrated upregulation of the X-linked TLR7 pathway member Tasl.
Conclusion: Our data demonstrate that perturbed XCI maintenance via Xist deletion in the B cell compartment results in the upregulation of X-linked Tasl, a contributor to abnormal immune signaling in SLE, along with activated B cell subset expansion, production of disease-specific autoantibodies, and glomerulonephritis. These findings suggest that disruption of XCI maintenance in B cells can both spontaneously confer and exacerbate SLE-like disease, thereby providing a novel pathogenic mechanism that simultaneously accounts for the strong female sex bias of SLE.
To cite this abstract in AMA style:
Lovell C, Jiwrajka N, Amerman H, Cancro M, Anguera M. Xist Deletion in B Cells Results in Systemic Lupus Erythematosus Phenotypes [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/xist-deletion-in-b-cells-results-in-systemic-lupus-erythematosus-phenotypes/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/xist-deletion-in-b-cells-results-in-systemic-lupus-erythematosus-phenotypes/