Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: It is standard practice to advocate for weight loss in patients with rheumatoid arthritis (RA) and obesity. Obesity and rheumatoid arthritis are both independently associated with cardiovascular disease. In addition, evidence suggests that obese RA patients are less likely to achieve remission and that weight loss may improve disease activity outcomes. Semaglutide and tirzepatide, both anti-obesity medications (AOM), were recently approved by the Food and Drug Administration (FDA) for treatment of obesity. However, there is currently a paucity of data on the clinical effects of AOM in RA patients.
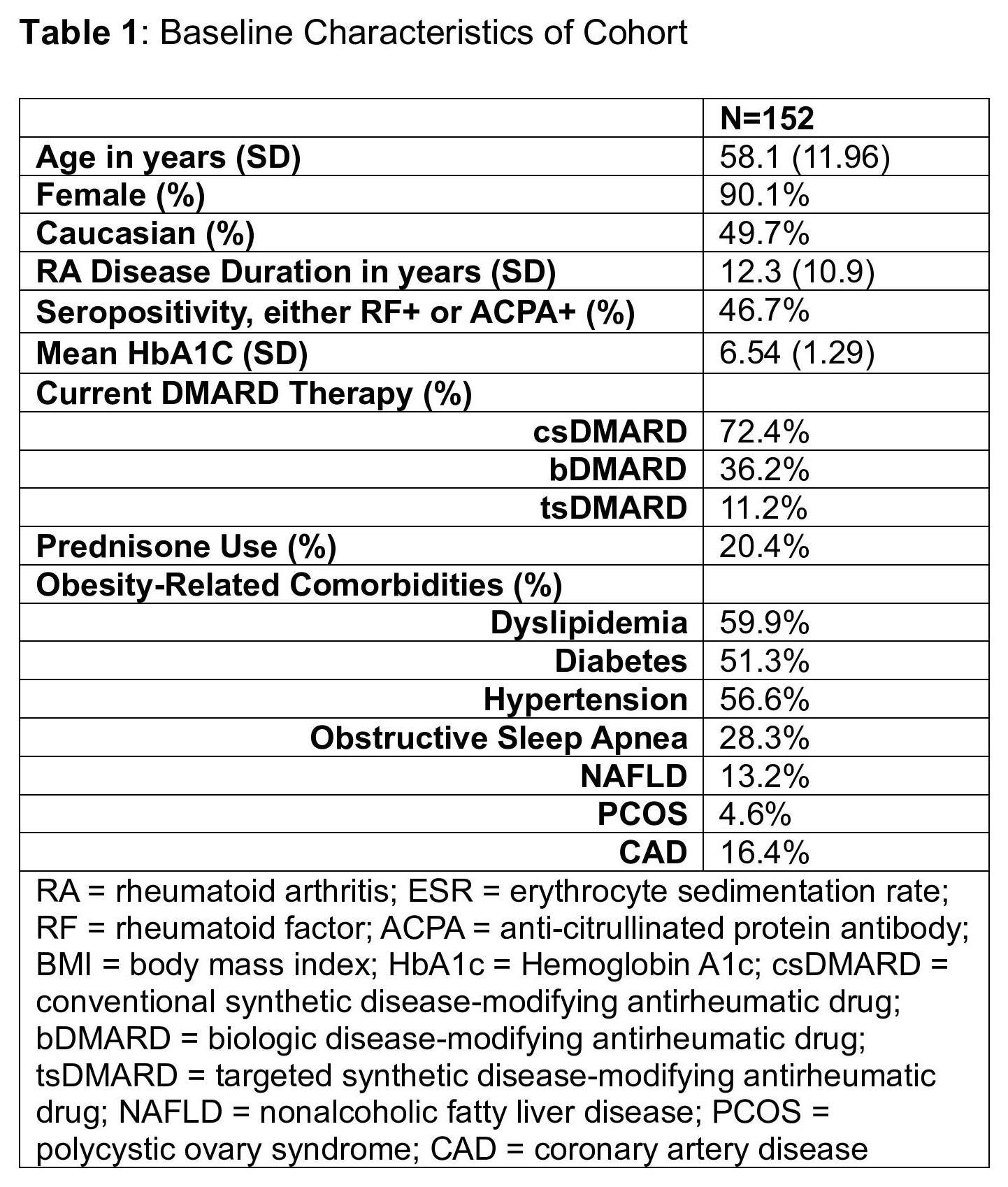
Methods: In this retrospective cohort study, we identified a group of patients with >1 RA diagnosis code in the electronic medical record who were prescribed an AOM (semaglutide or tirzepatide, either oral or subcutaneous). Detailed chart review of 554 patients revealed that 229 patients had RA (listed as a primary diagnosis in ≥2 rheumatology notes), and that 152 of these RA patients took the prescribed AOM. These patients’ charts were abstracted for demographics, weight, obesity-related comorbidities, current RA therapies, clinical and laboratory values, and adverse events at 3-month intervals for up to a year after starting AOM. There was significant incomplete data for composite disease activity RA outcomes. Changes in clinical and laboratory values over time were assessed using a linear mixed effects model where time was modeled as a fixed linear effect and the intercepts were modeled as random effects varying with subjects.
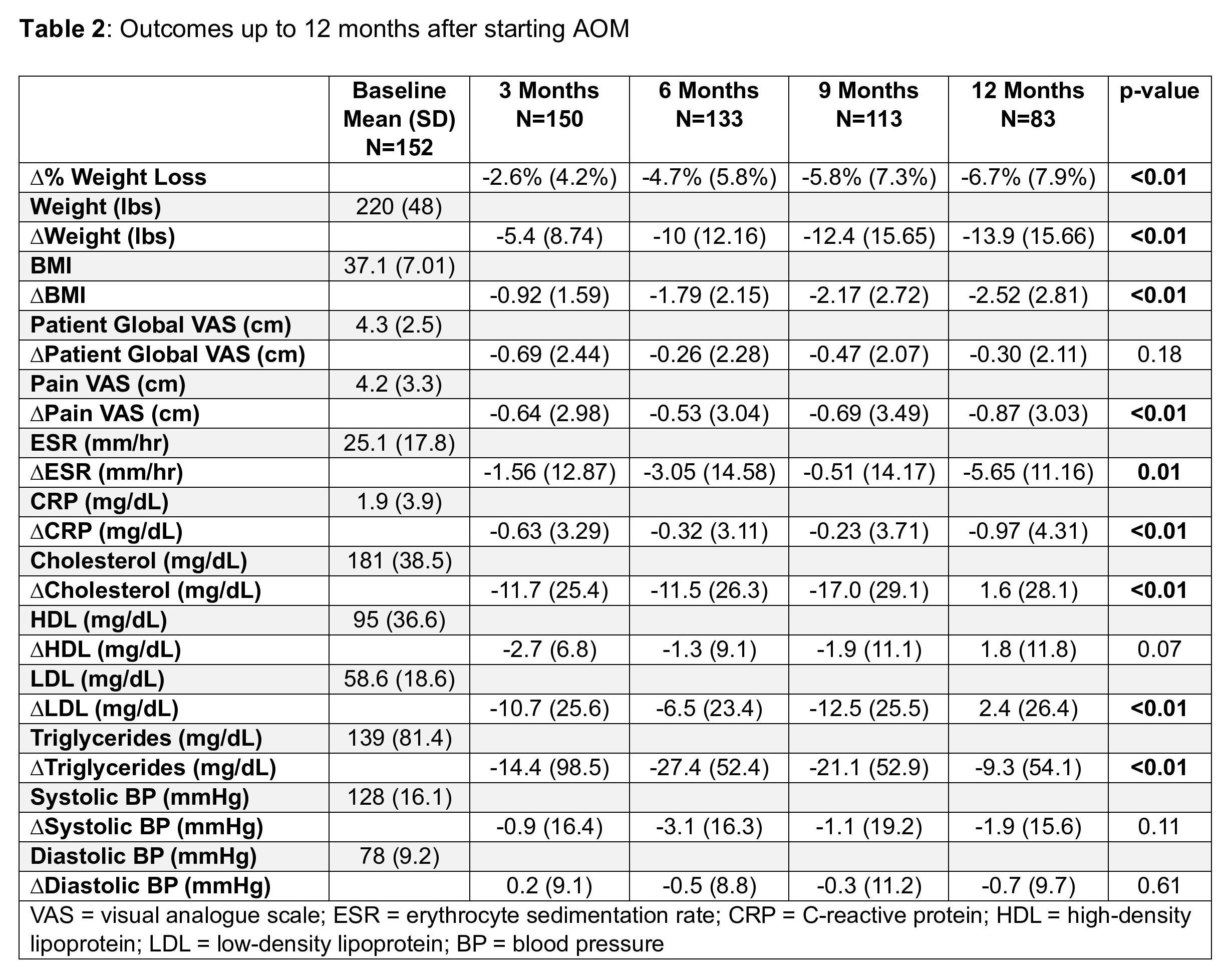
Results: The cohort had a mean age of 58.1 years and was predominantly female (90.1%). Obesity-related comorbid conditions were prevalent, including dyslipidemia (59.9%), diabetes (51.3%), hypertension (56.6%), and coronary artery disease (16.4%). RA patients were started on either semaglutide (93.4%) or tirzepatide (6.6%). There were significant reductions in weight, body mass index (BMI), acute phase reactants (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]), pain visual analogue scale (VAS), and lipids (total cholesterol, LDL cholesterol, and triglycerides) during the study period (Table 2). Approximately 15% of RA patients taking AOM had gastrointestinal side effects. There were 42 RA patients who discontinued AOM during the study period; the most common reasons for discontinuation were gastrointestinal side effects (23.8%), insurance issues (26.2%), and inefficacy (9.5%).
Conclusion: Our study suggests that the use of AOM may be associated with improvement in cardiovascular disease risks and pain within RA patients. Treatment with AOM was associated with significant weight loss as well as improvements in acute phase reactants (ESR and CRP), pain VAS, and lipids. The most common reasons for stopping AOM were gastrointestinal side effects and insurance issues. Further research is warranted to confirm the results of this retrospective study, and to examine RA disease activity outcomes.
To cite this abstract in AMA style:
Dente E, Kellner D, Tran V, Welsh T, Tran V, Saha A, Brook J, Elashoff D, Ranganath V. Effects of Anti-Obesity Medications in RA Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effects-of-anti-obesity-medications-in-ra-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-anti-obesity-medications-in-ra-patients/