Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Dermatomyositis (DM) is characterized by inflammatory and degenerative changes of skin and muscle and upregulation of Type I IFN-regulated gene and protein levels in the blood, muscle, skin, and endothelium. Type I IFN and other pro-inflammatory cytokines relevant to DM signal through the JAK-STAT pathway, and serum levels of IFN-β, IL-6, and IL-10 correlate with cutaneous disease activity, which is marked by exaggerated keratinocyte apoptosis and poses a significant unmet need for many patients. Brepocitinib is a novel TYK2/JAK1 inhibitor that has demonstrated efficacy at preventing Type I IFN-induced damage in human myotubes in vitro and may also be uniquely suited to improving skin manifestations by inhibiting IL-6, IFN-β, and IL-10 signaling via TYK2 and/or JAK1. Currently, once-daily (QD) oral brepocitinib (15 and 30 mg) is being evaluated in a double-blind, randomized, placebo-controlled Phase 3 study in patients with active DM (NCT05437263; VALOR Study).
Methods: To assess the potential of brepocitinib in treating cutaneous manifestations of DM, brepocitinib was used to treat IFN-stimulated normal human epidermal keratinocytes (HEKa). Briefly, HEKa were pretreated with 130 nM (unbound Cavg of 30 mg QD) or 1 μM brepocitinib for 2 hours and then stimulated with 1 ng/mL Type I IFN, 10 ng/mL IFN-γ, or both, for 24 hours. JAK-STAT signaling in keratinocytes was assessed via western blotting for phosphorylated STAT1 and STAT3; DM-relevant IL-6, IL-12, CXCL10 (IP-10), CCL2 (MCP-1), and ICAM-1 transcript levels were assessed with qPCR; and keratinocyte apoptosis was quantitated with flow cytometry via annexin V staining.
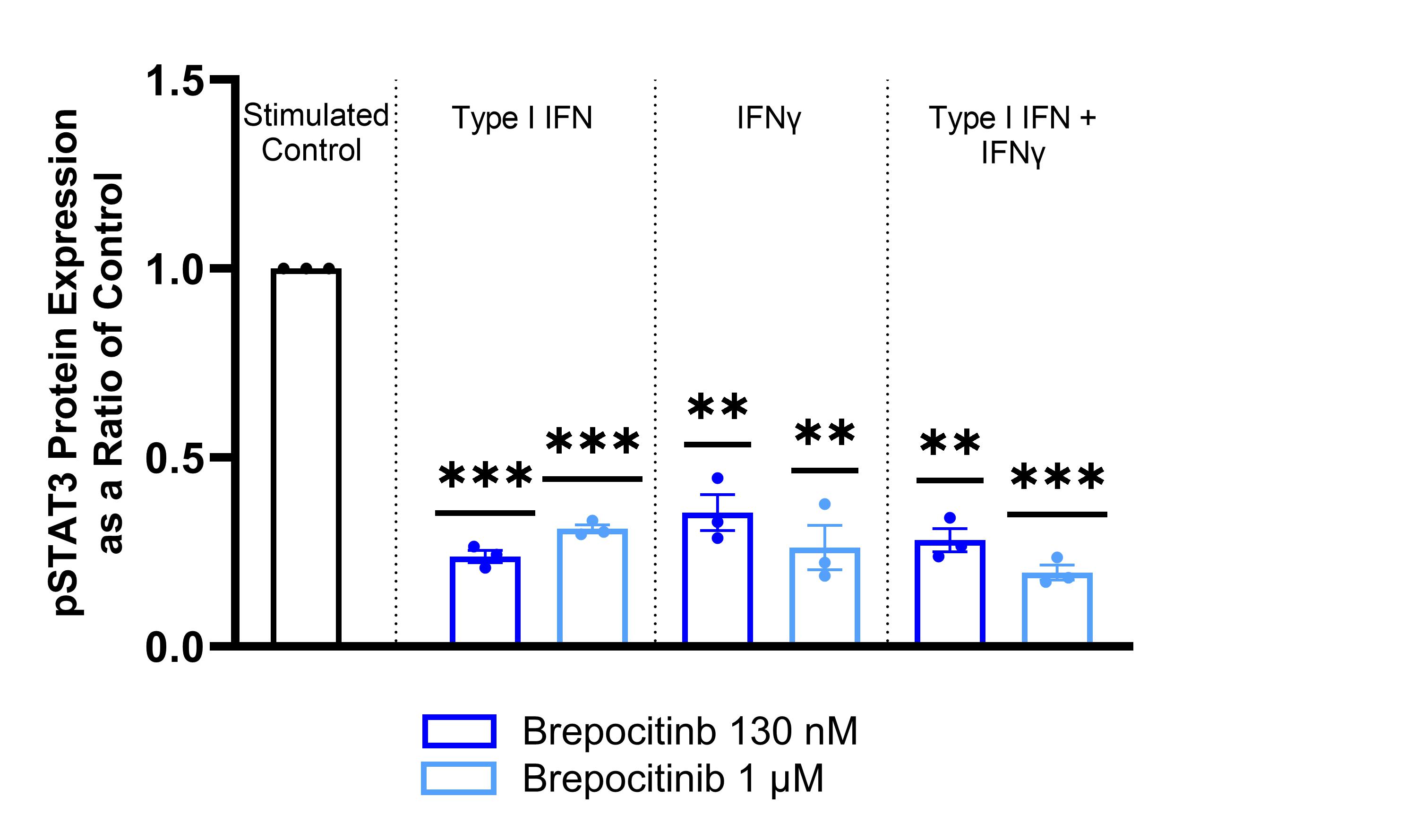
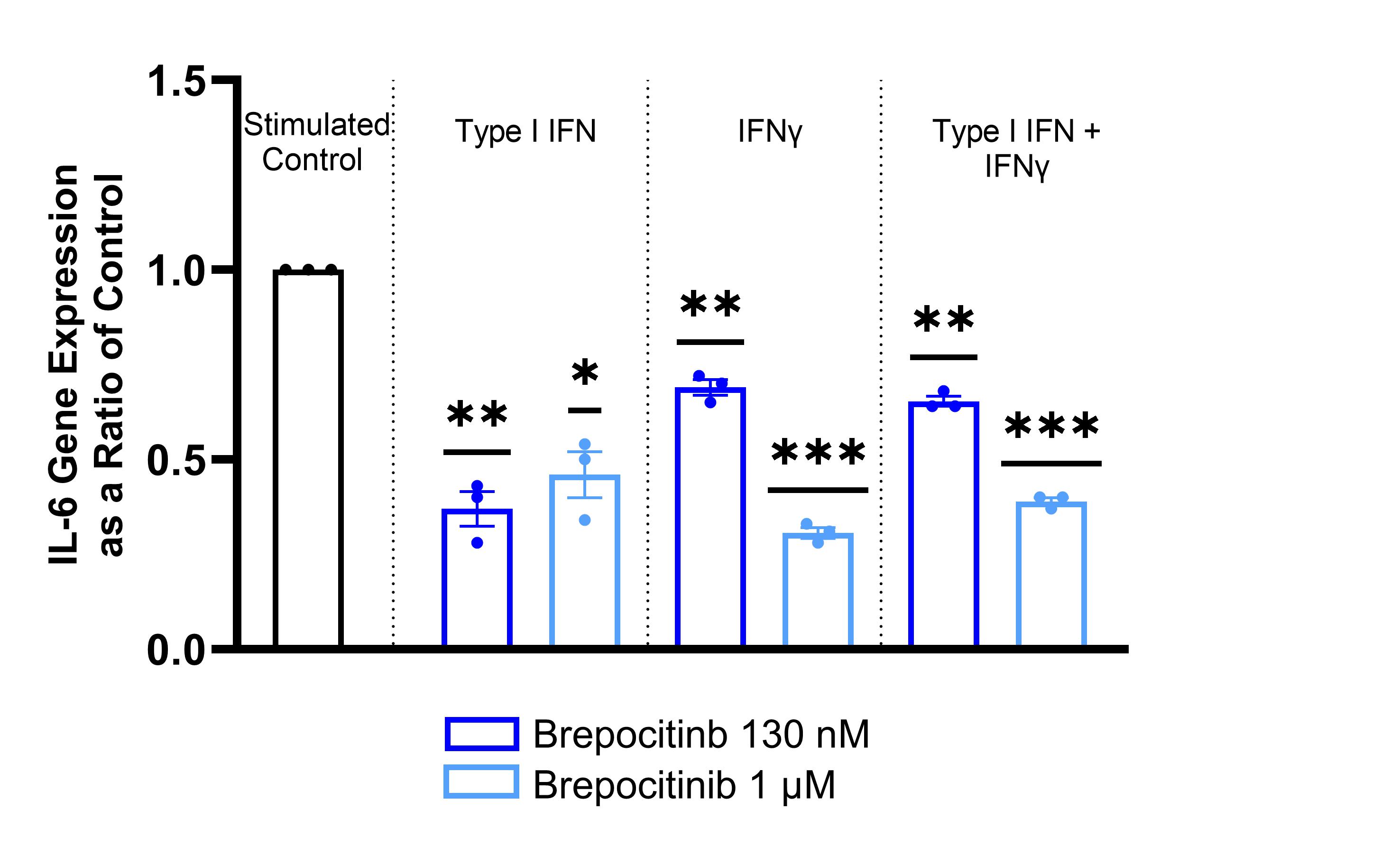
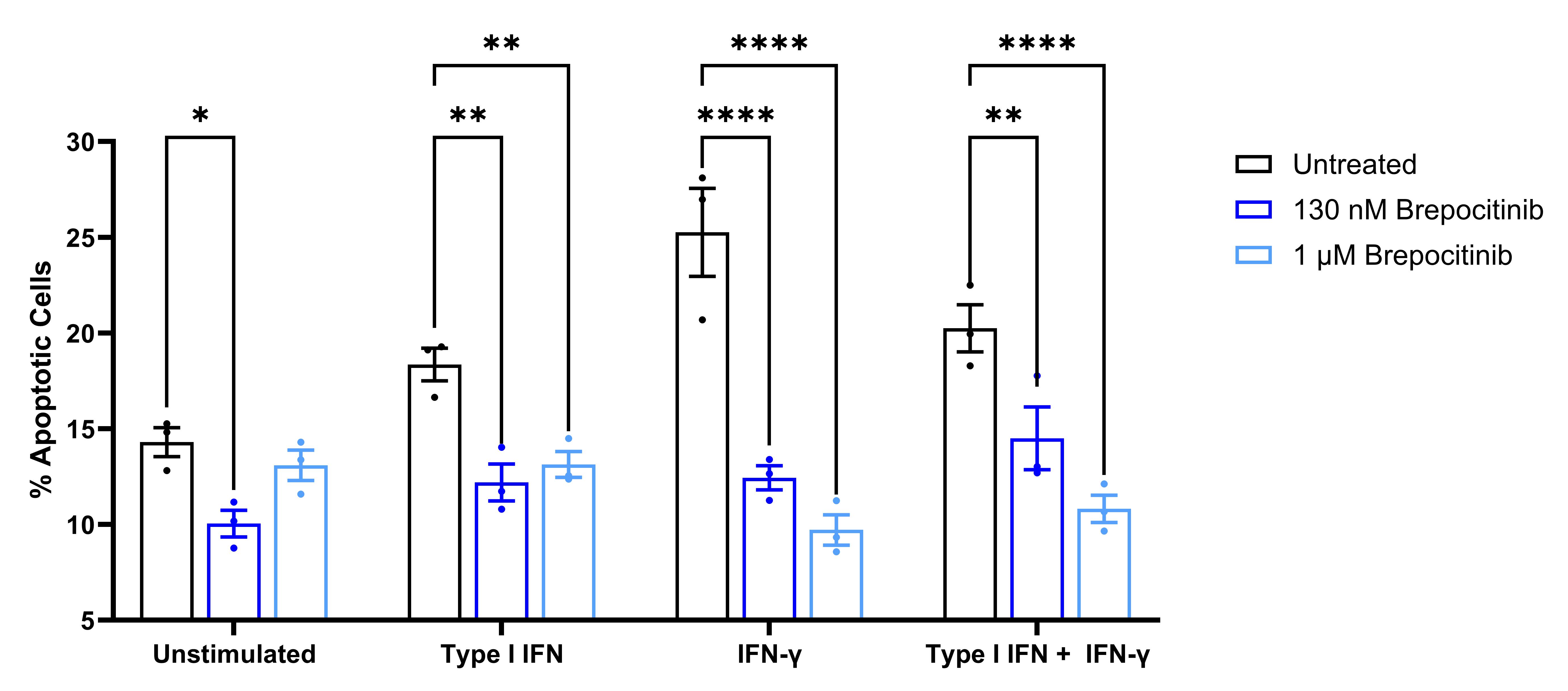
Results: Brepocitinib significantly reduced STAT3 phosphorylation in a concentration-dependent manner with all brepocitinib-treated phosphorylated STAT3 ratios between 0.20 and 0.35 of stimulated control (Figure 1). Brepocitinib 130 nM also significantly reduced STAT1 phosphorylation after stimulation with Type I IFN or IFNγ. Similarly, brepocitinib significantly reduced IL-6 gene expression in a concentration-dependent manner with brepocitinib-treated IL-6 expression levels between 0.31 and 0.69 of control (Figure 2). There were similar concentration-dependent reductions in IL-12, IP-10, MCP-1, and ICAM-1 gene expression levels. Finally, brepocitinib significantly reduced keratinocyte apoptosis and decreased the relative percentage of necrotic/late apoptotic cells by 28 to 61% depending on the stimulation condition (Figure 3).
Conclusion: Cutaneous disease activity in DM is marked by pro-inflammatory cytokine upregulation via the JAK-STAT pathway and aberrant keratinocyte apoptosis. Brepocitinib significantly reduced STAT1 and STAT3 phosphorylation in IFN-stimulated HEKa. In addition, brepocitinib led to concentration-dependent reductions in pro-inflammatory gene expression levels, including IL-6, and normalized apoptosis to basal levels in IFN-stimulated HEKa. These data provide further support for the potential of once-daily oral brepocitinib to be effective for the treatment of active DM, a hypothesis currently being tested in the ongoing Phase 3 VALOR study.
To cite this abstract in AMA style:
Vencovský J, Goriounova A, McConnachie L, Johnson B. Brepocitinib, a Selective TYK2/JAK1 Inhibitor Under Evaluation for the Treatment of Dermatomyositis, Reduces Inflammatory Cytokine Signaling and Interferon-induced Apoptosis in Primary Human Epidermal Keratinocytes [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/brepocitinib-a-selective-tyk2-jak1-inhibitor-under-evaluation-for-the-treatment-of-dermatomyositis-reduces-inflammatory-cytokine-signaling-and-interferon-induced-apoptosis-in-primary-human-epidermal/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/brepocitinib-a-selective-tyk2-jak1-inhibitor-under-evaluation-for-the-treatment-of-dermatomyositis-reduces-inflammatory-cytokine-signaling-and-interferon-induced-apoptosis-in-primary-human-epidermal/