Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Gut-residing bacteria, such as E.coli, can acetylate their proteome under conditions of amine starvation. It is postulated that the (gut) microbiome is involved in the breach of immune tolerance to modified self-proteins leading to the anti-modified protein antibodies (AMPA), hallmarking seropositive RA. Our aim was to determine whether acetylated bacterial proteins can induce AMPA-responses cross-reactive to modified self-proteins and be recognized by human AMPA (hAMPA).
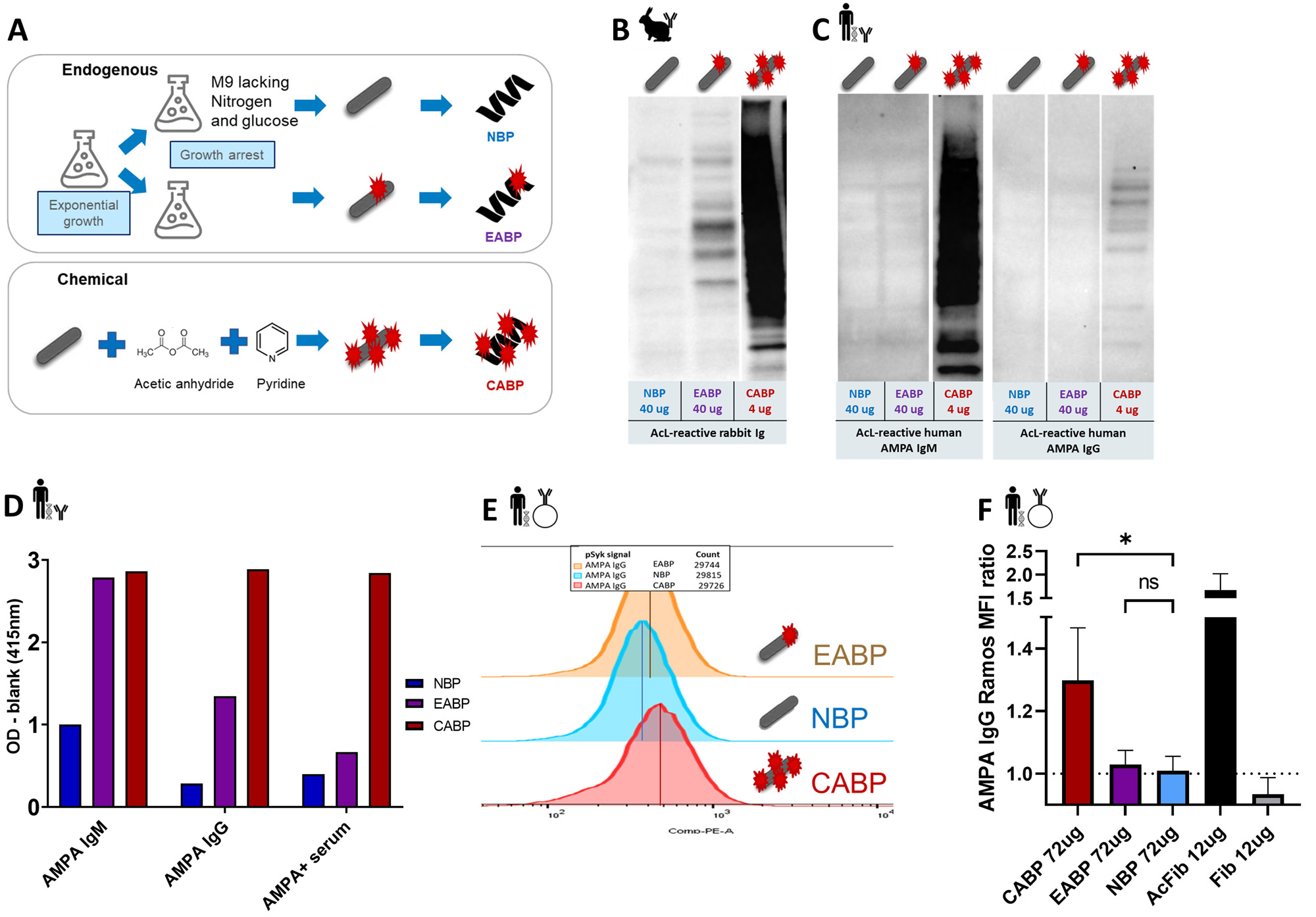
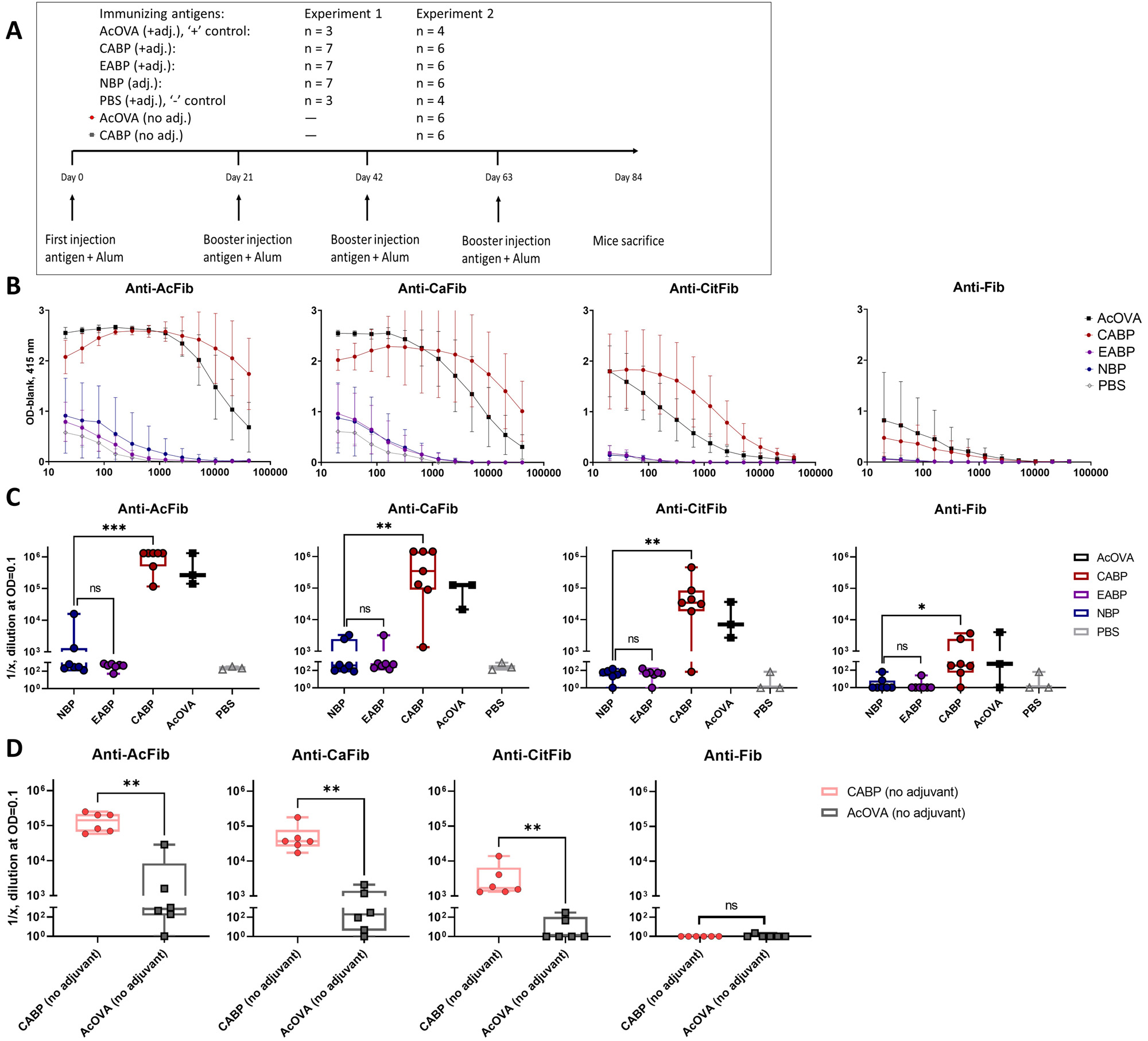
Methods: E. coli-bacteria were grown under amine starvation to generate endogenously acetylated bacterial proteins. Furthermore, E. coli proteins were acetylated chemically (Figure 1A). Recognition of these proteins by hAMPA was analysed by western blotting and ELISA; recognition by B cells carrying a modified protein-reactive B cell receptor (BCR) was analysed with a pSyk activation assay. C57BL/6 mice were immunized with (modified) bacterial protein fractions, and sera were analysed by ELISA (Figure 2A).
Results: Chemically modified bacterial protein fractions contained high levels of acetylated proteins (Figure 1B) and were readily recognized by hAMPA (Figure 1C) and able to activate B cells carrying modified protein-reactive BCRs (Figure 1E-F). Likewise, although expressing lower levels of acetylation, also endogenously acetylated protein fractions were recognized by hAMPA in ELISA (Figure 1D). Immunizing mice with chemically modifed protein fractions induced a strong cross-reactive AMPA response, targeting various modified antigens including citrullinated proteins (Figure 2B-C). Interestingly, highly acetylated bacterial proteins could induce an AMPA response even without an adjuvant (Figure 2D).
Conclusion: Acetylated bacterial proteins are recognizable by hAMPA and capable of inducing a cross-reactive AMPA response in mice. These observations provide first evidence for a novel mechanism, involving the (endogenous) acetylation of the bacterial proteome, allowing a breach of tolerance to modified proteins and the formation of cross-reactive AMPA.
(A) Schematic overview of the generation of NBP, EABP and CABP. (B) Western blotting with NBP, EABP and CABP stained with anti-acetyllysine polyclonal rabbit antibody. (C) Western blotting with NBP, EABP and CABP stained with hAMPA mAbs, recognizing acetylated proteins: 7E4 IgG and 1E3 IgM. (D) ELISA with 1E3 IgM, 7E4 IgG and polyclonal IgG from an RA patient serum isolated with CAcP4 peptide. (E) pSyk MFI, visualizing BCR activation of Ramos B cells expressing 7E4 IgG after incubation with acetylated E. coli antigens. (F) Summary of three BCR activation experiments with 7E4 Ramos B cells. Statistical differences are indicated with asterisks, indicating p-value: * < 0.05, ** < 0.01, **** < 0.0001.
(A) Immunization scheme of the performed mouse experiments. (B) Reactivity of titrated mouse serum to modified and unmodified versions of fibrinogen, per immunizing antigen. (C) Mice immunized with the antigens with the adjuvant: titers at OD = 0.1, as determined by ELISA to modified and native versions of fibrinogen. (D) Mice immunized with CABP or AcOVA without the adjuvant: reactivity of individual mouse serum samples to modified and unmodified versions of fibrinogen. Statistical differences are indicated with asterisks, indicating p-value: * < 0.05, ** < 0.01, **** < 0.0001.
A-C: data are representative of two experiments. AcOVA: acetylated ovalbumin, CABP: chemically acetylated bacterial proteins; EABP: endogenously acetylated bacterial proteins; NBP: non-acetylated bacterial proteins; PBS: phosphate buffer saline.
To cite this abstract in AMA style:
Volkov M, Kampstra A, van Schie K, Kwekkeboom J, de Ru A, van Veelen P, Huizinga T, Toes R, van der Woude D. Acetylated Bacterial Proteins as Potent Antigens Inducing an Anti-modified Protein Antibody Response [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/acetylated-bacterial-proteins-as-potent-antigens-inducing-an-anti-modified-protein-antibody-response/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/acetylated-bacterial-proteins-as-potent-antigens-inducing-an-anti-modified-protein-antibody-response/