Session Information
Date: Tuesday, November 14, 2023
Title: Plenary III
Session Type: Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: As part of the Accelerating Medicines Partnership (AMP), we discovered that urinary PR3, a neutrophil degranulation product, is associated with histological activity, indicating the involvement of neutrophil degranulation in proliferative LN, the most aggressive type of LN. Although mature neutrophils with classical polylobate nuclei are rare in LN kidney biopsies, recent evidence suggests that immature myeloid cells undergoing degranulation play a role in the pathogenesis of LN. Our aim is to investigate the presence of PR3+ cells in LN kidney, their association with histopathological features and to define their immunophenotype.
Methods: We performed multiplexed histology using serial immunohistochemistry (sIHC) on LN kidney biopsies to quantify the expression of PR3 and multiple cell lineage markers (20-plex). Image analysis including deconvolution, cell segmentation, glomerular annotation, and quantitative histology was performed using Indica HALO. The analysis was limited to renal cortex.
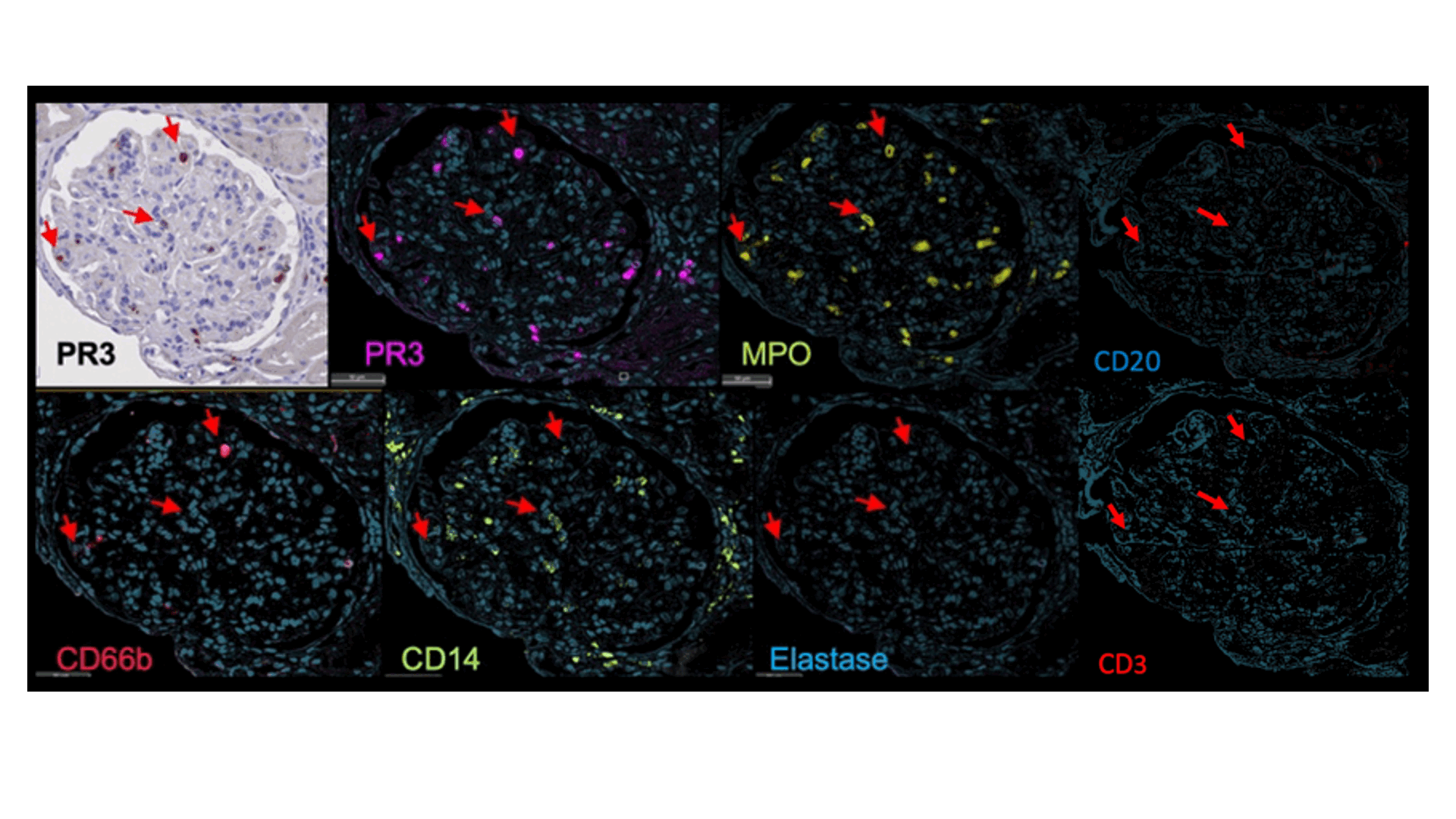
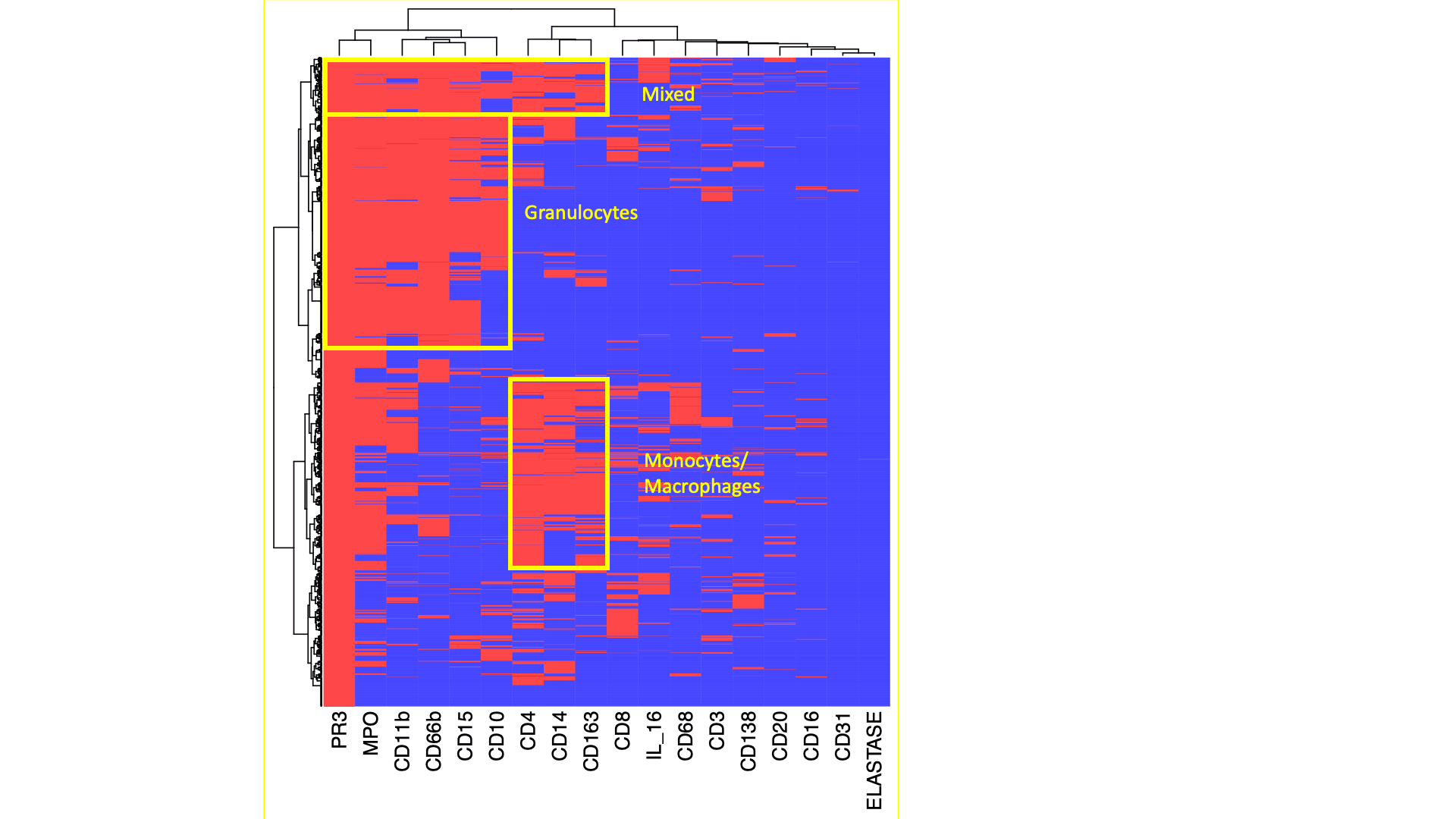
Results: A total of 11 patients with LN who underwent a clinically indicated kidney biopsy were enrolled: 6 (55%) with pure proliferative LN (ISN/RPS class III or IV) and 5 (45%) with pure membranous LN. PR3+ cells were identified in all biopsies (range 343-7625 per sample) and most of them did not show a polylobate nucleus. The majority of PR3+ cells were in the tubulointersitium (Figure 1A). However, when present, there was a higher density of PR3+ cells in the glomeruli due to their smaller area(Figure 1A-C). PR3+ cell abundance was higher in proliferative LN, especially in the glomeruli (Figure 1A-C). Glomerular PR3+ cell density very strongly correlated with histological activity measured by the NIH Activity Index (Pearson’s r=0.97, p=5*10-5; Figure 1D). PR3+ cells displayed a unilobate nucleus (Figure 2). Most PR3+ cells coexpressed MPO. We identified 2 subsets of PR3+ cells based the coexpression of other lineage markers (Figures 2 and 3). One group coexpressed CD66b, CD11b, CD15, and (variably) CD10 indicating a degranulating phenotype (Figure 3). The other group expressed CD14, CD163, and CD4 indicating a phagocytic phenotype(Figure 3).The was a smaller subset with features of both groups.
Conclusion: PR3+ cells are abundant in LN, increased in proliferative LN and are strongly associated with histological activity thereby characterizing a more aggressive phenotype. This population densely infiltrated the glomeruli emphasizing a potential role in the endothelial pathogenic process. There were two subsets of kidney-infiltrating PR3+ cells characterized by a phagocytic or a degranulating phenotype; both were mononucleated suggesting a monocyte or nonsegmented neutrophil lineage. We previously showed the association between urinary PR3 and histological activity suggesting that intrarenal PR3+ cells are actively degranulating and therefore likely inducing kidney damage. These findings nominate PR3+ cells as a potential therapeutic target. Spatial transcriptomics and proteomic studies are ongoing to define the lineage and function of these cells.
The original and deconvoluted IHC images are shown for a representative glomerulus, with arrows indicating three identical representative cells. Markers indicated at the bottom with matching false colors.
Heatmaps displays the heterogeneity of marker coexpression at the single cell level fo PR3+ cells. Three distinct subsets of PR3+ cells infiltrating the kidney were identified. Data from one representative kidney biopsy (class IV lupus nephritis, NIH Activity Index 8/24, Chronicity Index 6/12).
To cite this abstract in AMA style:
Celia A, Yang X, Minsky H, Malvica S, Petri M, Accelerating Medicines Partnership in RA/SLE t, Rosenberg A, Fava A. Degranulating PR3+ Myeloid Cells Characterize Proliferative Lupus Nephritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/degranulating-pr3-myeloid-cells-characterize-proliferative-lupus-nephritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/degranulating-pr3-myeloid-cells-characterize-proliferative-lupus-nephritis/