Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICI) are an increasingly used form of anti-cancer therapy, but they have been associated with a range of cardiac immune-related adverse events (iRAEs). Early detection of cardiac iRAEs may lead to prompt intervention and improved patient outcomes. In this study, we investigate the association between regular troponin monitoring and major adverse cardiac events (MACE) detection and overall survival (OS) in patients receiving ICI.
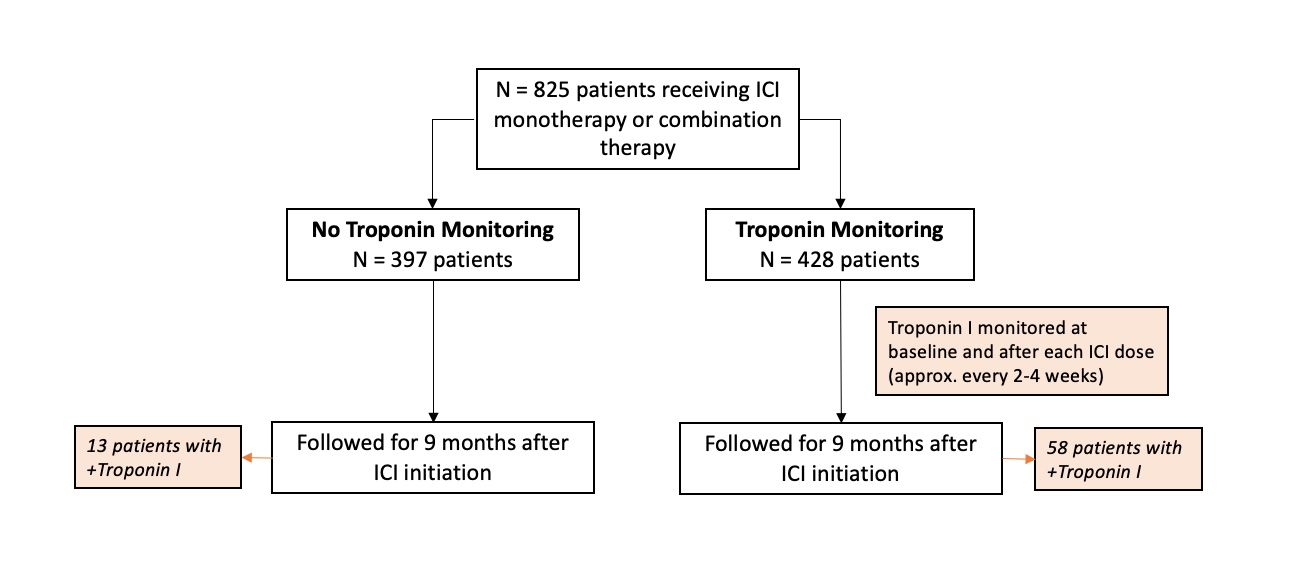
Methods: We performed a retrospective study of 825 patients receiving ICI from January 2018 to March 2022. Of these patients, 428 (52%) were evaluated via a standard troponin monitoring protocol with Troponin I measured at baseline and prior to each ICI dose. The control group included 397 (48%) patients who underwent ICI therapy prior to initiation of this troponin monitoring protocol. We collected outcomes in all patients for nine months following their first dose of ICI. Primary outcomes included severe MACE (defined as grade ≥ 4 Cardiovascular Adverse Events (CV AEs) according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 5) and OS. To investigate whether troponin monitoring was associated with discontinuation of ICI therapy, we compared time-to-treatment-failure (TTF) between monitored and unmonitored patients in a subgroup of patients with melanoma. TTF was defined as the interval from ICI initiation to discontinuation for any reason. Additionally, we studied the relationship between troponin elevation and MACE by comparing the rates of MACE (defined as grade ≥3 CV AEs) between all patients with a positive troponin measurement during the monitoring period versus patients with negative or no troponin measurement. A positive troponin was defined as Troponin I >0.055.
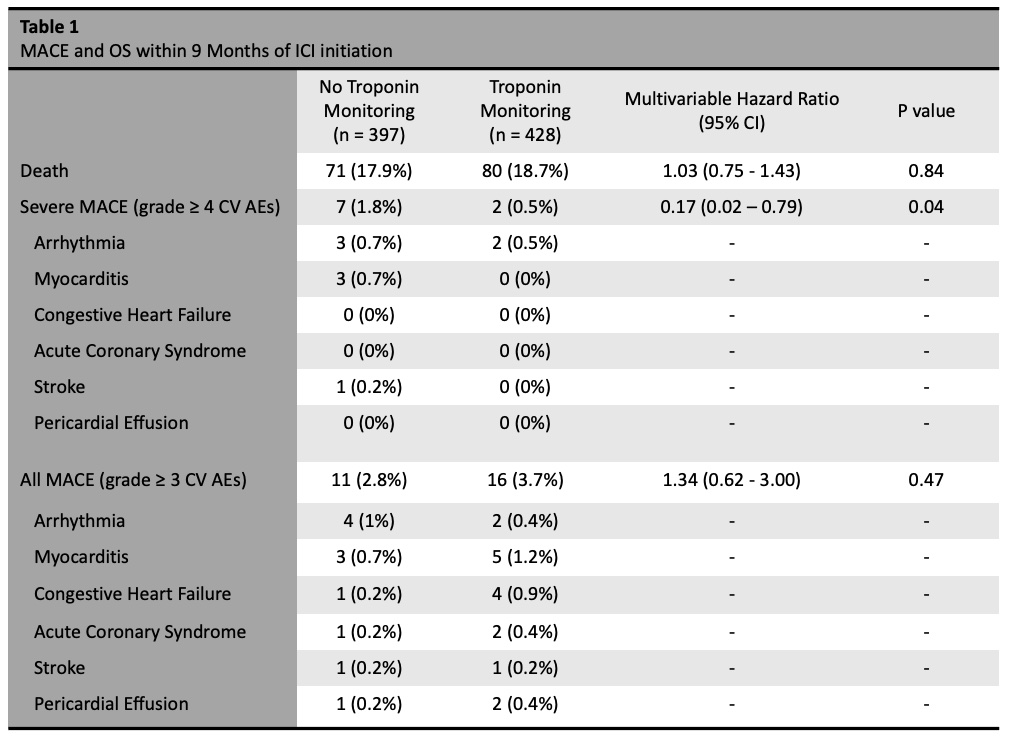
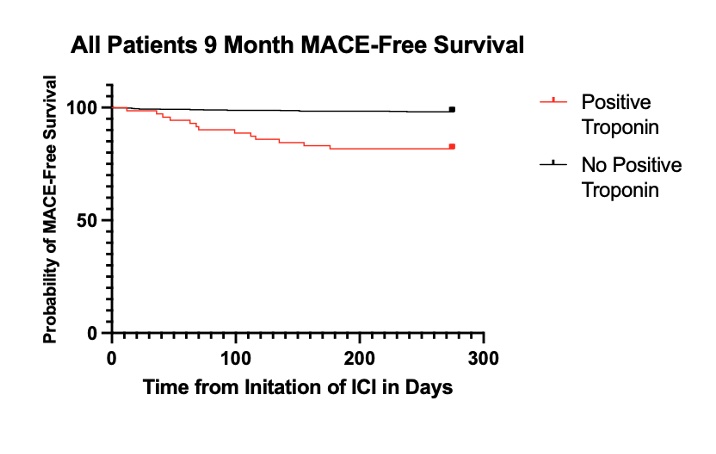
Results: We found a lower rate of severe MACE in patients who underwent troponin monitoring (0.5%) compared to patients who underwent no troponin monitoring (1.8%), (HR 0.17, 95% CI 0.02-0.79). There was no difference in OS at nine months between monitored patients (81%) and unmonitored patients (82%), (HR 1.04, 95% CI 0.75-1.44). In patients with melanoma (N=89), we found no difference in TTF at nine months between patients who were monitored (54% discontinued ICI) and unmonitored (53% discontinued ICI) (p = 0.91). There were 71 patients (8.6%) with positive troponin measurement during the evaluated interval. Patients with positive troponin had a higher risk of MACE compared to those with negative or no troponin measurement (HR 7.20, 95% CI 2.99–17.55). This finding remained consistent when evaluated in the monitored subgroup alone (HR 4.34, 95% CI 1.578 – 12.38).
Conclusion: We found that patients undergoing regular troponin monitoring with ICI therapy had a lower rate of severe MACE compared to those without monitoring. Troponin elevation was significantly associated with MACE. Additionally, there was no difference in OS between those who were monitoring or unmonitored, and there was no difference in TTF between these two groups in patients with melanoma.
no difference in all MACE and overall survival between the two groups.
To cite this abstract in AMA style:
Ivanovic M, Chan A, Franquiz M, Xu S, Lee C, Fazal M, You J, Witteles R, Neal J, Wu S, Waliany S, Zhu H. Impact of Troponin Monitoring on Cardiac Outcomes in Patients Receiving Immune Checkpoint Inhibitors [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/impact-of-troponin-monitoring-on-cardiac-outcomes-in-patients-receiving-immune-checkpoint-inhibitors/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-troponin-monitoring-on-cardiac-outcomes-in-patients-receiving-immune-checkpoint-inhibitors/