Session Information
Date: Monday, November 13, 2023
Title: (0859–0885) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Pentosan Polysulfate sodium (PPS) is a semi-synthetic polysaccharide and a glycosaminoglycan mimetic. The multiple actions of PPS involve anti-inflammatory effects via inhibition of NF-κB; analgesia by normalising the pain mediator, NGF; and chondroprotection by inhibiting ADAMTS-5 degradation of aggrecan in cartilage. The study objectives were to evaluate the durability of effect of PPS therapy in companion dogs with naturally occurring osteoarthritis (OA) at six months (26 weeks) and to evaluate the effects of PPS for disease modifying potential.
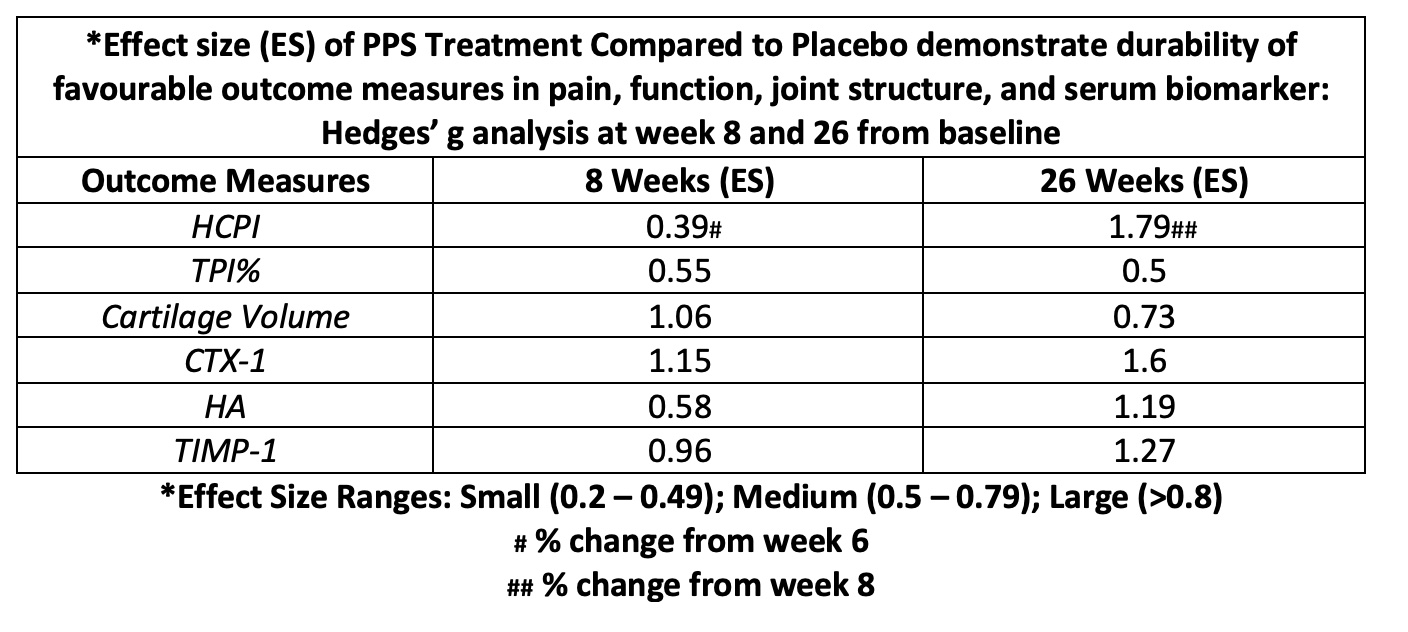
Methods: In a double-blinded study, twenty dogs (12 males and 8 females; mean weight: 26.05 kg, (SD 4.5); mean age: 9.9 years, (SD 2.0)) with OA were randomised to subcutaneous PPS injections (3 mg/kg; human equivalent dose of 1.7 mg/kg) or placebo weekly for 6 weeks in a 2:1 ratio, respectively. At baseline, week 8 and week 26, the index stifle or elbow were analysed by the Helsinki Chronic Pain Index (HCPI) for pain; gait analysis by Total Pressure Index % (TPI%) for joint function; and total cartilage volume by MRI for joint structural changes. Validated ELISAs for serum biomarkers of joint degeneration (CTX-I, Hyaluronic acid (HA), TIMP-1) were assayed. The mean percentage change from baseline (%CFB) at weeks 8 and 26 were used to determine the effect size (ES) of PPS treatment compared to placebo using the Hedges’ g test.
Results: PPS treatment showed meaningful ES improvement in pain at week 26 compared to placebo with a reduction in mean percentage change from the previous follow-up at week 8 in HCPI (ES 1.79). The improvement in pain was reflected by a larger effect size at the later time of 26 weeks relative to week 8 (ES 0.39) suggesting durability of PPS effects on pain. Long-term functional improvements in gait were demonstrated by an increase in mean %CFB in TPI% and sustained by ES of 0.55 and 0.5 at weeks 8 and 26, respectively. Furthermore, the mean %CFB in TPI% for PPS treatment at weeks 8 and 26 was 7.3% and 8.2%, respectively, whereas clinically meaningful increases reported at 5% were not met by placebo. There were larger reductions in cartilage volume in the placebo group compared to PPS treatment at 8 and 26 weeks with a large PPS ES of 1.06 and 0.73, respectively suggesting reduction in structural degeneration of the articular cartilage. PPS favourably affected serum levels of CTX-1, HA and TIMP-1 supporting the in vivo mechanisms of drug action.
Conclusion: Compared to placebo, the long-term effect of PPS therapy results in the improvement of pain, function, cartilage volume and serum biomarkers at 26 weeks from baseline suggesting that PPS stabilises disease progression in canine OA and may translate to disease modification in humans. PPS was well tolerated during treatment and follow up.
To cite this abstract in AMA style:
Krishnan R, Stapledon C, Reiter C, Ryan S, Bauquier S, Beths T. Pentosan Polysulfate Sodium, a Glycosaminoglycan Mimetic Demonstrates Durable Effects on Pain, Function and Joint Structure in Canine Naturally Occurring Osteoarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/pentosan-polysulfate-sodium-a-glycosaminoglycan-mimetic-demonstrates-durable-effects-on-pain-function-and-joint-structure-in-canine-naturally-occurring-osteoarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pentosan-polysulfate-sodium-a-glycosaminoglycan-mimetic-demonstrates-durable-effects-on-pain-function-and-joint-structure-in-canine-naturally-occurring-osteoarthritis/