Session Information
Date: Sunday, November 12, 2023
Title: (0066–0095) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: A role for CD4 T cells in RA pathology is supported by the effectiveness of T cell therapies, genetic studies implicating T cell gene regulation, and association with specific MHC Class II HLA-DRB1 alleles. Using expansive healthy cohorts, Ishigaki et al (Nat Gen 54:393-402 (2022)) previously identified CD4 T cell receptor (TCR) repertoire features associated with the presence of HLA-DRB1 RA risk alleles. These features can be combined into a TCR risk score. This TCR risk score included a positive association with high risk HLA alleles and specific amino acids in certain positions of the CDR3 region (for example, negative charged residues at position 110). Here, we computed TCR repertoire risk scores for 12 RA patients. Understanding these properties would have implications for our conception of the underpinnings of RA pathology.
Methods: We obtained synovial tissue (n=8) and blood (n=8) from patients satisfying either the 1987 or 2010 ACR/EULAR RA classification criteria, with informed consent and IRB approval. We flow-sorted CD4 T cells and applied single-cell transcriptome and T-cell receptor sequencing (10X platform). We used our custom computational pipeline, implemented with scanpy, to assign CD4 transcriptional subtypes. We quantified TCR repertoire including clonal expansion and the Ishigaki et al TCR risk score, We also inferred HLA-DRB1 genotypes (using arcasHLA) from which HLA-RA risk scores were computed. ~5,000-9,000 cells per sample were analyzed.
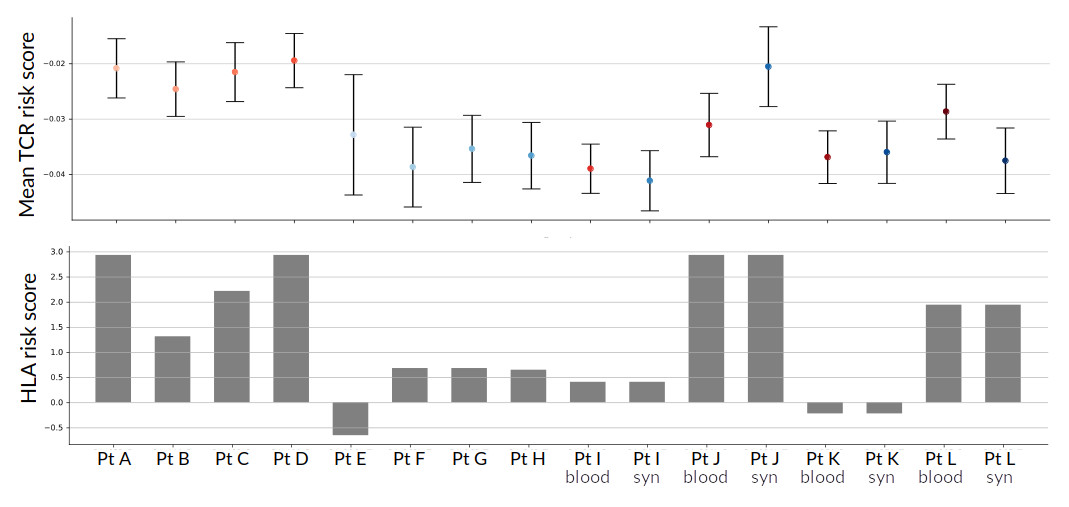
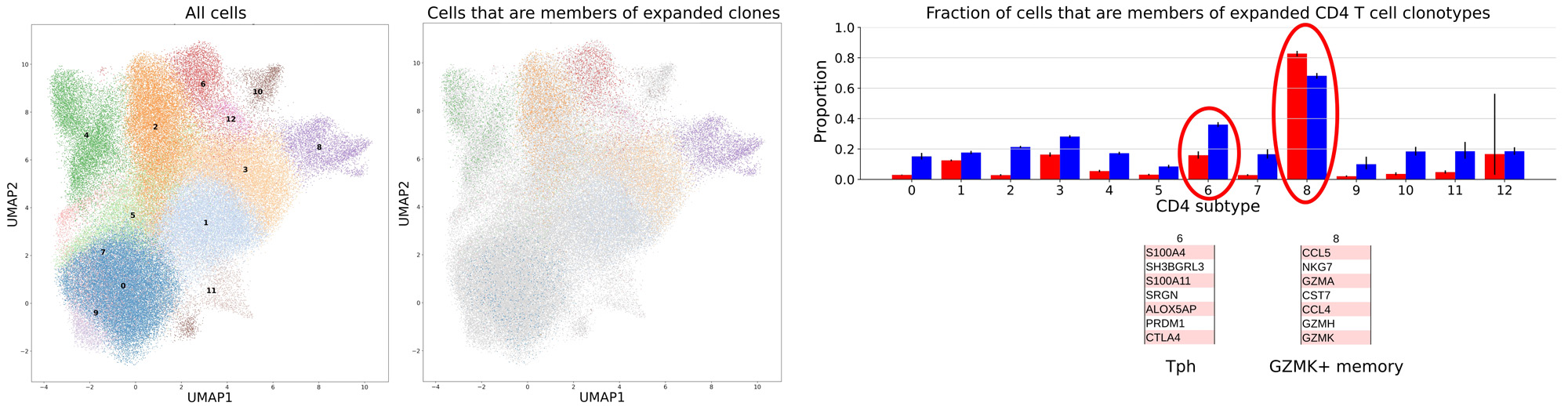
Results: Across 12 RA patients, the TCR repertoire risk score positively correlated with the HLA-RA genetic risk score in each subject [Fig 1]; the degree of association is similar to that seen in the large-scale healthy cohorts. Three patients had the highest possible HLA-risk score (~3.0), due to both HLA-DRB1 copies containing the highest scoring risk alleles, which were all shared-epitope alleles. Notably, these patients also demonstrated the highest TCR risk score across their repertoires. For patients from which we had paired blood and synovial tissue CD4 T cells, the patient with the maximal HLA risk score demonstrated a larger overall TCR repertoire risk score in the synovium. Examining features of clonal CD4 T cell populations, we noted a preponderance of cells from expanded clones within two specific CD4 subsets, the T peripheral helper (Tph) and GZMK+ memory CD4 cells [Fig 2].
Conclusion: Our study has defined TCR receptor repertoire patterns that associate with high-risk HLA-DRB1 alleles in RA patients. Indeed, we now show that having two risky HLA-DRB1 alleles increases the total number of risk-associating T cell clones. Given the low frequency of clone sizes for CD4 T cells in general, the total number of risky T cell clonotypes may be a new marker of increased RA risk and argue for a broader repertoire-wide view of CD4 T cell involvement in RA rather than singular antigenic focused hypotheses. Future studies on these particular clones in high-risk repertoires may become highly relevant in further understanding RA disease etiology and treatment.
Shades of red in the upper plot denote a blood origin of the sample, while shades of blue denote a synovial origin.
Across all samples, there is a general tendency toward higher mean TCR risk score with higher HLA risk score.
Looking within the paired samples (the rightmost 8 bars), there is no consistent relationship between the TCR risk score based on blood or synovial origin of the CD4 cells
The table summarizes genes found to be characteristic of the CD4 subtypes in which the most clonal expansion was observed, based on the scRNAseq data (circled in bar plot above, blue bars for synovium, red bars for blood). Based on those marker genes, two of the CD4 subtypes are identifiable as previously distinguished populations, T peripheral helper cells (Tph) and GZMK+ memory CD4 cells
To cite this abstract in AMA style:
Lakhanpal A, Ishigaki K, Singaraju A, Kochen A, Fein M, Raychaudhuri S, Donlin L. CD4 T Cell Repertoire Features in RA Patients with High-risk HLA-DRB1 Alleles [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cd4-t-cell-repertoire-features-in-ra-patients-with-high-risk-hla-drb1-alleles/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd4-t-cell-repertoire-features-in-ra-patients-with-high-risk-hla-drb1-alleles/