Session Information
Date: Sunday, November 12, 2023
Title: (0066–0095) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Activated T cells make a significant contribution to inflammation in systemic lupus erythematosus (SLE). We know that cellular metabolism regulates the activation of T cells. Evidence from patients with multiple sclerosis indicates that dimethyl fumarate (DMF), an electrophile, targets cellular metabolism to modulate CD4+T cell activation and function.
Here, we investigated whether DMF modulates T cell metabolism and function in samples from patients with SLE in a series of in vitro experiments.
Methods: All experiments were performed using isolated T cells from freshly drawn whole blood samples from patients with SLE. T cells were isolated using negative selection using Stem cell or Miltenyei magnetic bead separation kit. Isolated T cells were activated with anti-CD3 and IL-2 and incubated with either DMF at 25μM concentration or DMSO alone for three or seven days at 37°C and 5% CO2 before harvesting.
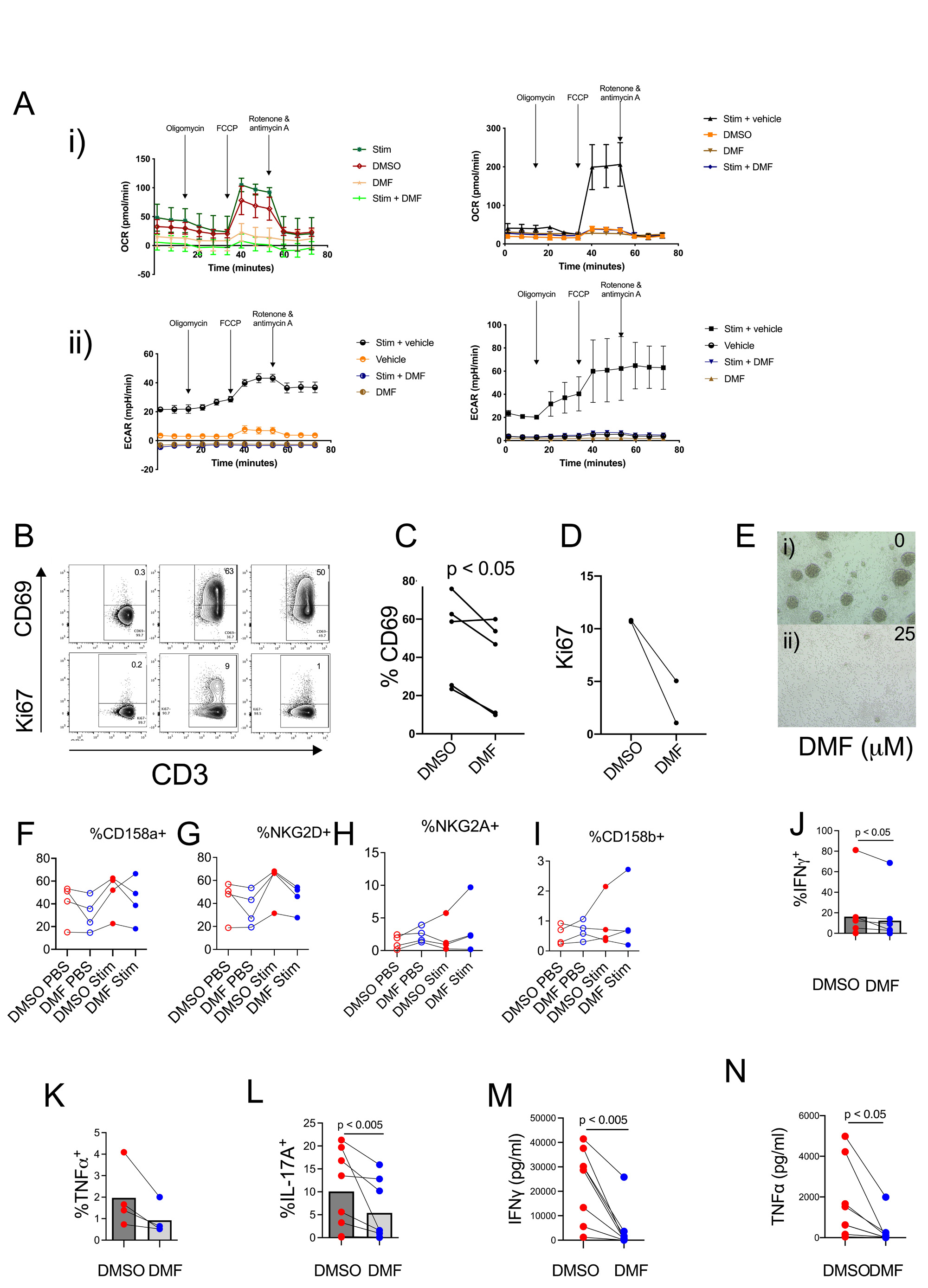
Results: In Seahorse experiments, after three days of incubation dimethyl fumarate (DMF) inhibited Figure A-i) the oxygen consumption rate (OCR) and A-ii) extra cellular acidification rate (ECAR) in isolated T cells when compared with samples incubated with vehicle, dimethyl sulfoxide (DMSO). Our results revealed that DMF significantly inhibited: 1) aerobic glycolysis and oxidative phosphorylation in activated T cells from patients with SLE (n=4) (Figure A- i and ii), in vitro; 2) T cell activation and proliferation as assessed by a reduction in the frequency of CD69 (n=4) and D) Ki67 (n=2) positivity, respectively (Figure B-E); 3) Collectively, these results suggest that DMF inhibits T cell activation and proliferation in samples from patients with SLE.
After 7 days of incubation, DMF significantly inhibited the expression of activating NK receptors CD158a and NKG2D on T cells whereas there was a trend toward enhancing the expression of inhibitory NK receptors NKG2A and CD158b (Figure F-I).
After 7 days of incubation, DMF significantly reduced intracellular expression of IFN-γ, TNF-α, IL-17 and secretion of pro-inflammatory cytokines IFN-γ and TNF-α in supernatants (Figure J – N).
Conclusion: Our preliminary data indicated that DMF modulates metabolic programming to inhibit activation, proliferation, and secretion of proinflammatory cytokines from T cells patients with SLE.
A-I) In Seahorse experiments, after three days of incubation dimethyl fumarate (DMF) inhibited the oxygen consumption rate (OCR) and A-ii) extra cellular acidification rate (ECAR) in isolated T cells when compared with samples incubated with vehicle, dimethyl sulfoxide (DMSO). These results revealed that DMF significantly inhibited aerobic glycolysis and oxidative phosphorylation in activated T cells from patients with SLE (n=4), in vitro.
B) Flow cytometry gating strategy to analyse for activated T cells CD3+CD69+ and proliferating T cells CD3+Ki67+. C) After three days of incubation, DMF significantly inhibited T cell activation and proliferation as assessed by a reduction in the frequency of CD69 (n=4) and D) Ki67 (n=2) positivity, respectively. E-i) After 7 days of incubation, the density of T cells at 10 X magnification in flat bottom wells without DMF was higher than E-ii) in the well in the presence of DMF. Collectively, these results suggest that DMF inhibits T cell activation and proliferation in samples from patients with SLE. After 7 days of incubation, DMF significantly inhibited the expression of activating NK receptors F) CD158a and G) NKG2D on T cells whereas DMF seemed to have a trend toward enhancing the expression of inhibitory NK receptors H) NKG2A and I) CD158b.
After 7 days of incubation, DMF significantly reduced intracellular expression of J) IFN-γ, K) TNF-α, L) IL_17 and secretion of pro-inflammatory cytokines M) IFN-γ and N) TNF-α in supernatants.
Thus our preliminary data indicated that DMF modulates metabolic programming to inhibit activation, proliferation and secretion of proinflammatory cytokines from T cells patients with SLE.
To cite this abstract in AMA style:
Kell L, Taylor S, Shah K, De Maeyer R, Sen D, Castelino M, Cambridge J, Isenberg D, Leandro M, Akbar A, Reddy v. Dimethyl Fumarate Modulates T Cell Metabolism and Function in Systemic Lupus Erythematosus Patient Samples [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/dimethyl-fumarate-modulates-t-cell-metabolism-and-function-in-systemic-lupus-erythematosus-patient-samples/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dimethyl-fumarate-modulates-t-cell-metabolism-and-function-in-systemic-lupus-erythematosus-patient-samples/