Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Immune checkpoint inhibitors represent a major advance in cancer treatment, but disease prognosis continues to be poor for most patients. Resistance of cancer cells to lymphocyte killing and appearance of autoimmune toxicities restrict dosing and duration of therapy. Here we present CD6, a cell surface glycoprotein expressed by T-lymphocytes and human natural killer (NK) cells, that engages in cell-cell interactions by binding to its ligands CD166 and CD318 (CDCP1). Interrupting CD6-CD318 interaction with the monoclonal anti-CD6 antibody termed UMCD6, reduces Th1- and Th17-driven autoimmune responses in CD6-humanized mice, conferring beneficial effects in several mouse models of autoimmunity and inflammation. Recently, we have demonstrated that UMCD6 enhances CD8+ T and NK cells to kill multiple cancer lines. We now seek to explore the efficacy of UMCD6 in vivo and understand how cancer cells interact with immune cells to control the immune response to cancer.
Methods: Subcutaneous xenograft study. To investigate the long-term efficacy of UMCD6 in vivo, breast cancer cells (MDA-MB-231) were injected subcutaneously into SCID/beige mice. Mice were infused with 107 human PBMC or 106 NK cells once and treated with 100µg of UMCD6, anti-PD-1 or control IgG antibody weekly. Tumor volume was measured by bioluminescence imaging. Flow cytometry was used to define phenotypic changes in tumor infiltrating lymphocytes from breast cancer xenografts.
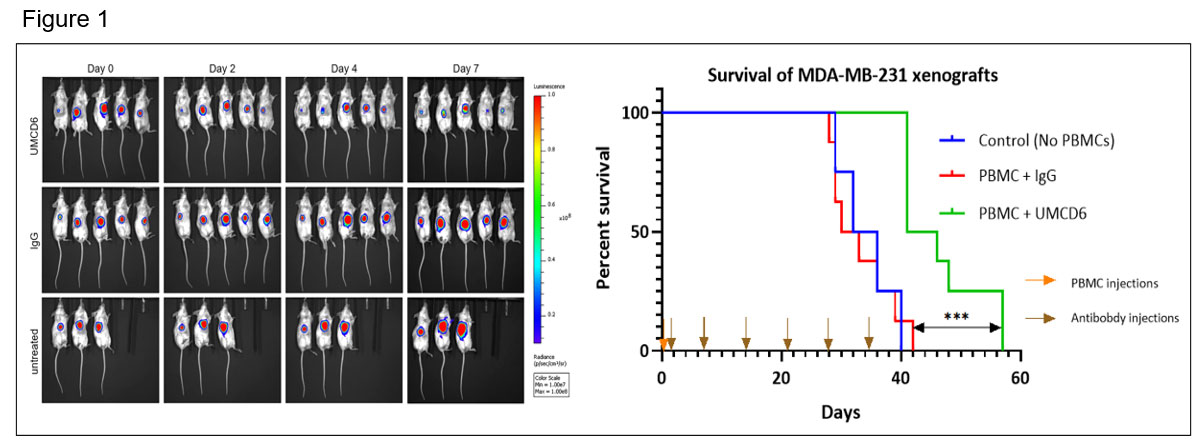
Results: UMCD6 increases survival and augments killing by human PBMC and NK cells of a human breast cancer line xeno-transplanted into immunodeficient mice. Tumor growth was significantly reduced by UMCD6 from day 7 and maintained until at least day 28 (*p< 0.05) (figure 1). At day 38, a robust increase in survival can be seen in the UMCD6 treated mice (100% alive, n=8) compared to IgG (25% alive, n=8) and control (25% alive; n=4). UMCD6 also decreased tumor growth and increased survival in mice infused with human NK cells (*p< 0.05) (figure 2).
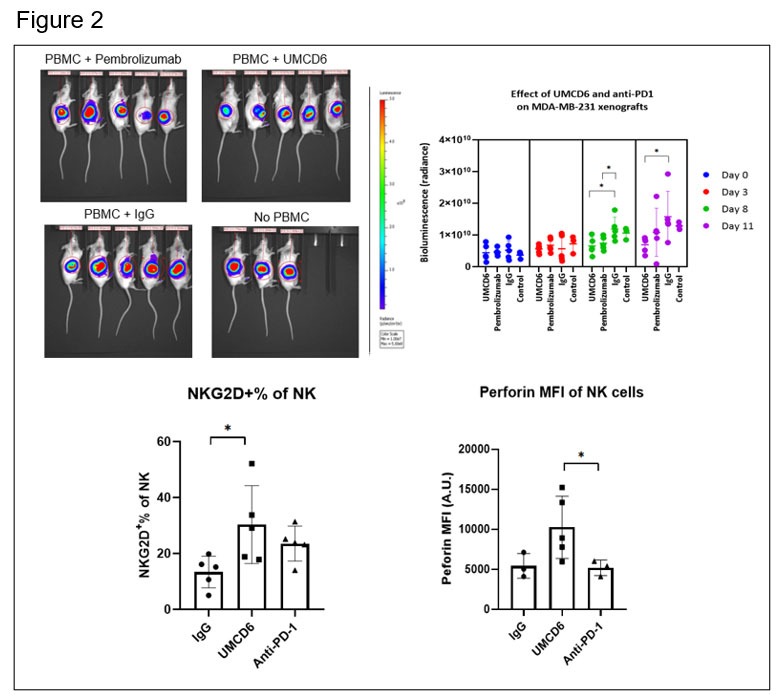
The effect of UMCD6 is more sustained in killing of MDA–MB–231-derived xenograft tumors by PBMC than anti-PD-1. Both UMCD6 and pembrolizumab enhanced tumor killing of PBMC at day 8 compared to IgG control (*p< 0.05), but only UMCD6 showed statistical significance at day 11. Tumor tissues immunostained for CD56 from mice administered UMCD6 showed activated NK cells that were associated with areas of decreased density of tumor cells. Evaluation of Tumor Infiltrating Lymphocytes (TILs) revealed a higher frequency of NK cells in mice treated with UMCD6. The expression of NKG2D, an activating NK receptor, and perforin, was also increased in NK and NKT cells from mice receiving UMCD6. Additionally, UMCD6 modulated the expression of multiple NK receptors, cytokines and genes that regulate the tumor microenvironment.
Conclusion: Our antibody UMCD6 effectively reduces several mouse models of autoimmunity thorough effects on CD4+ lymphocyte differentiation and enhances in vivo killing of breast cancer cells through direct activation of NK and CD8 cells. These data point to a potential new and safer approach to cancer immunotherapy that would suppress rather than instigate autoimmunity.
To cite this abstract in AMA style:
Gurrea-Rubio M, Wu Q, Tsou E, Amin M, Campbell P, Randon P, Lind M, Ory S, Amarista C, Vichaikul S, Ruth J, Ling F, Fox D. Targeting CD6-CD318 Axis with UMCD6 (anti-CD6) Enhances in Vivo Killing of Cancer Cells Through Direct Activation of NK Cells [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/targeting-cd6-cd318-axis-with-umcd6-anti-cd6-enhances-in-vivo-killing-of-cancer-cells-through-direct-activation-of-nk-cells/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-cd6-cd318-axis-with-umcd6-anti-cd6-enhances-in-vivo-killing-of-cancer-cells-through-direct-activation-of-nk-cells/