Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Systemic lupus erythematosus (SLE) is characterized by a defective T regulatory (Treg) cell compartment which participate in immune dysregulation. Although the underlying mechanism are unknown, dysregulated T cell metabolism has been widely reported in SLE, and its normalization leads to disease improvement (1). Calcium/calmodulin dependent protein kinase (CaMK4) activity is increased in SLE and has been shown to affect T cell metabolism (2). Our objective was to investigate the mechanisms underlying Treg cell metabolic dysregulation in SLE, with a focus on CaMK4.
Methods: We harvested CD62L+CD4+ T cells from wild-type (WT) or Camk4-/- mice and differentiated them in vitro into inducible Treg (iTreg) cells. We evaluated iTreg metabolism using Seahorse XF analyzer and mass spectrometry (metabolomics). Phosphofructokinase activity was assessed by a colorimetric assay (Abcam). In vitro gene knockdown was conducted by transfecting a guide RNA (gRNA) in CRISPR/Cas9-expressing T cells. Treg cell function was evaluated by in vitro immunosuppressive assay and in vivo by the adoptive transfer of T conventional T and iTreg cells (8:1 ratio) in Rag1-/- mice to induce inflammatory colitis. The relevance of CaMK4 in SLE was evaluated in vivo using a T-cell specific knockdown of CaMK4 in the B6.lpr mouse model, and in humans by culturing SLE patient T cells with KN-93, a CaMK4 specific inhibitor.
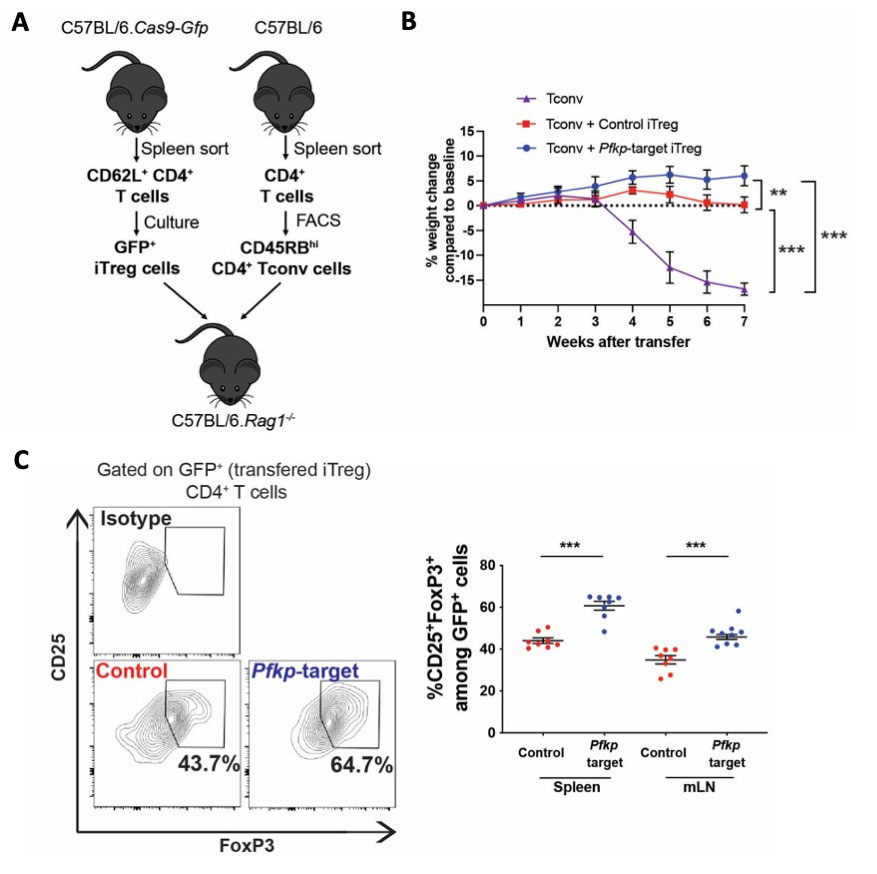
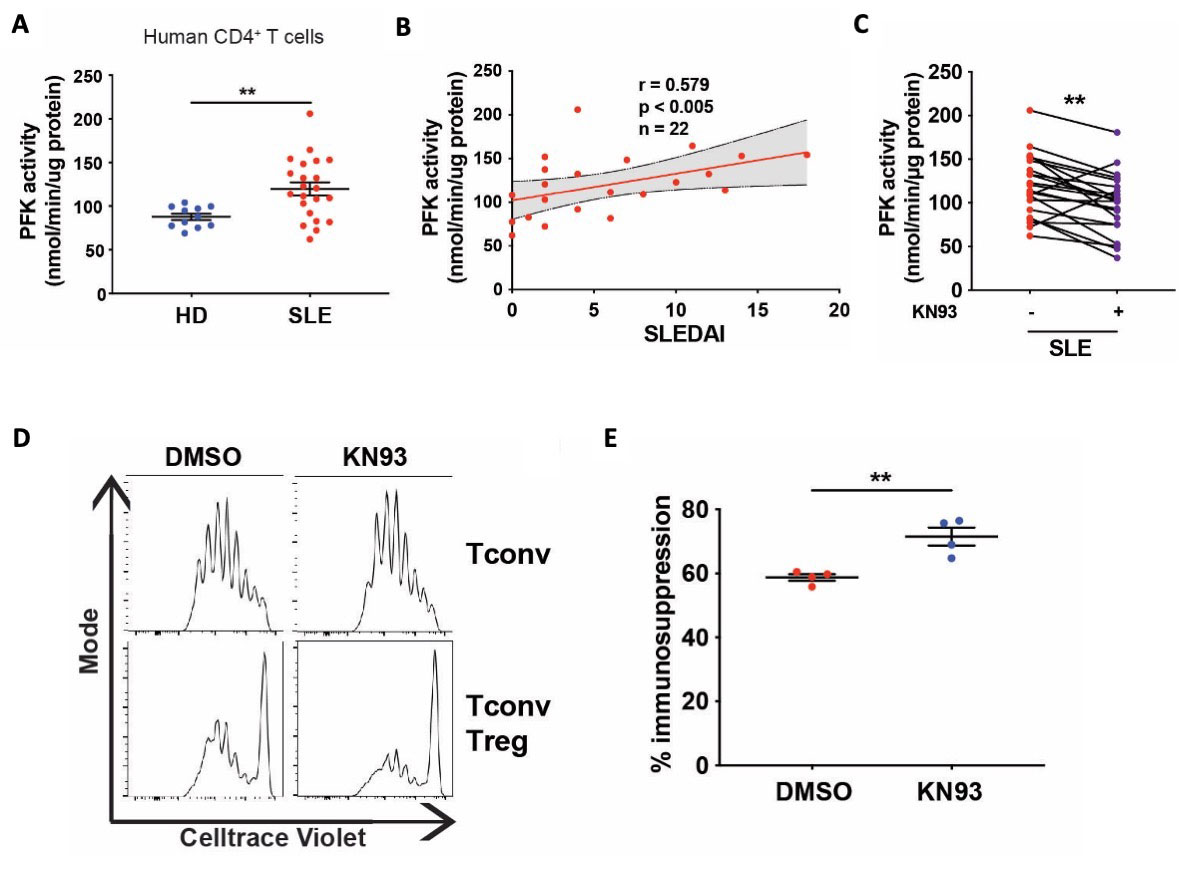
Results: iTreg cells from Camk4-/- mice had decreased glycolysis and increased mitochondrial metabolism compared to WT mice. Metabolomics studies suggested decreased activity of the rate-limiting glycolysis enzyme phosphofructokinase platelet-type (PFKP). While PFKP mRNA and protein levels were similar between WT and Camk4-/- iTreg, PFKP activity was significantly decreased in Camk4-/- iTreg, suggesting a post-transcriptional control of PFKP activity by CaMK4. Mechanistically, immunoprecipitation experiments confirmed that CaMK4 interacted with PFKP, and phosphoproteomic study suggested that CaMK4 phosphorylated serine residue 539 of PFKP, a site known to control PFKP activity. To confirm the importance of PFKP in Treg biology, we showed that PFKP knockdown significantly improved iTreg function in vitro (p < 0.01) and in vivo using an adoptive colitis model (Fig. 1A-B). In vivo, iTreg lacking PFKP were less likely to lose FoxP3 expression (Fig. 1C) and to produce IL-17A, demonstrating higher Treg stability in an inflammatory environment. On a translational basis, lupus-prone B6.lpr mice with a T-cell specific CaMK4 knockdown displayed improved disease pathology. Human SLE CD4+ T cells had higher PFKP activity compared to healthy donors, and PFKP activity correlated with the SLE disease activity index (SLEDAI, r = 0.579, p < 0.005; Fig. 2A-B). Finally, culture of SLE CD4+ T cells with KN-93 led to a significant decrease in PFKP activity (p < 0.001, Fig. 2C), and an improvement of Treg cell immunosuppressive activity (Fig. 2D-E).
Conclusion: By fine-tuning their immunometabolism, PFKP controls the immunosuppressive function and stability of Treg cell in SLE, and represents a promising therapeutic target.
References:
1. Yin Y, et al. Sci Transl Med. 7(274):274ra18.
2. Kono M, et al. JCI Insight 4(12):e127395.
(A) Design of the in vivo experiment. Cas9-GFP expressing CD62L+ CD4+ T cells were transfected with control of Pfkp-target sgRNA and differentiated into iTreg cells. CD45RBhi CD4+ T (Tconv) cells were sorted using FACS from CD4+ T cells presorted from the splenocytes of C57Bl/6 mice. T-deficient RAG1-/- mice were transferred with 4×106 Tconv and 5×105 iTreg. Data are from 3 independent experiments of 2_3 mice per condition. (B) Weight of mice transferred with Tconv cells only (purple, n = 4), Tconv and control iTreg cells (red, n = 8) or Tconv and Pfkp-target iTreg cells (blue, n = 8). After sacrifice, the percentage of CD25+FoxP3+ Treg cells was evaluated among initially transferred iTreg identified as GFP+ CD4+ T cells, in the spleen and mesenteric lymph nodes (mLN; n = 8). Points and bars represent an individual value and s.e.m., respectively. **, p < 0.01; ***, p < 0.001.
(A) PFK activity was measured from the lysate of CD4+ T cells cultured during 24 hours with CD3/CD28 activation. Samples from HD (n = 11) and SLE patients (n = 22). (B) Spearman correlation of the CD4+ T cells PFK activity and the SLE disease activity index (SLEDAI; n = 22).The delimited grey area indicates the 95% confidence interval of the linear regression. (C) PFK activity was measured from the lysate of CD4+ T cells cultured during 24 hours with CD3/CD28 activation with or without KN93 (10μM; n = 22). (D) Representative results of Tconv cells proliferation. (F) The percentage of immunosuppression was calculated by comparing Treg/Tconv cell proliferation to the Tconv cell proliferation from the corresponding condition (n = 4 biological replicates). Points and bars represent an individual value and s.e.m., respectively. **, p < 0.01.
To cite this abstract in AMA style:
Scherlinger M, Pan W, Hisada R, Vukelic M, Umeda M, Boulougoura A, Tsokos M, Tsokos G. Phosphofructokinase P Fine-Tunes T Regulatory Cell Metabolism, Function and Stability in Systemic Autoimmunity [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/phosphofructokinase-p-fine-tunes-t-regulatory-cell-metabolism-function-and-stability-in-systemic-autoimmunity/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phosphofructokinase-p-fine-tunes-t-regulatory-cell-metabolism-function-and-stability-in-systemic-autoimmunity/