Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Synovial lining fibroblasts secrete factors into the joint cavity that promote joint lubrication1. In inflammatory arthritis, sublining fibroblasts undergo marked expansion while lining fibroblasts are lost, leading to joint damage2,3,4. While we previously reported the role of Notch signaling in driving sublining fibroblast differentiation, molecular signals driving lining fibroblasts remain unknown.
Methods:
- Patient-derived organoids. We developed an en-block, patient-derived organoid to culture synovial tissue that retains fibroblast heterogeneity ex vivo.
- Micromass model. We resuspended cultured synovial fibroblasts in Matrigel to create an in vitro 3D model.
- Phenotypic analyses. We employed live imaging of organoids, histology, and single-cell RNA sequencing (scRNAseq) to characterize fibroblast phenotypes.
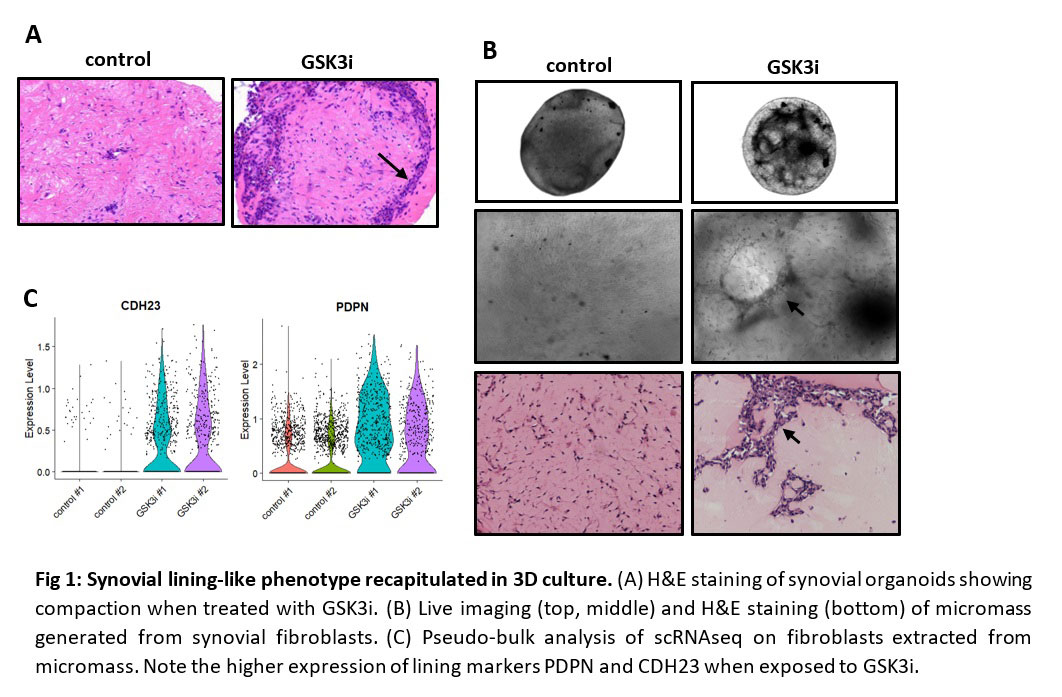
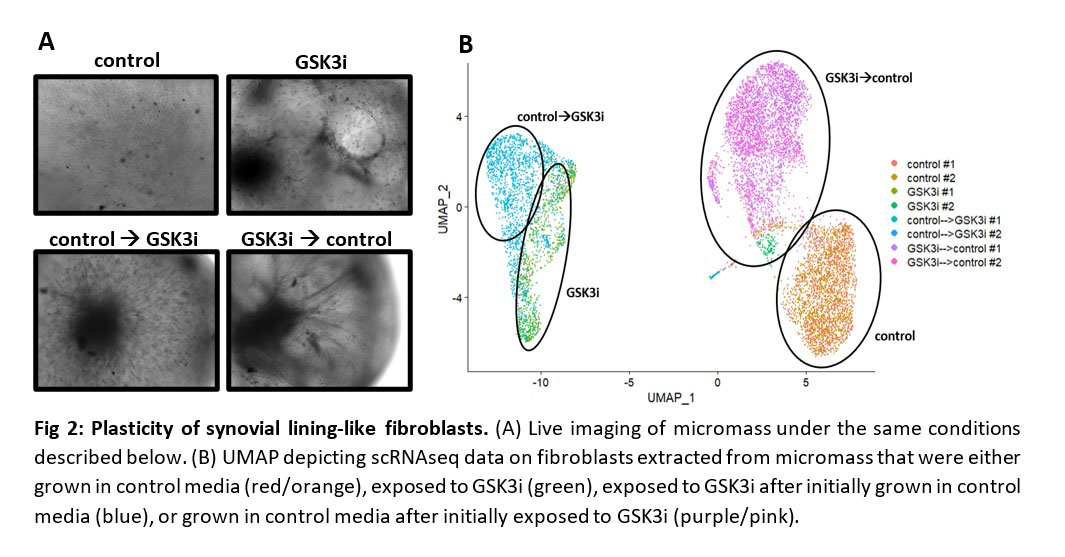
Results: We unexpectedly observed that the addition of a glycogen synthase kinase 3 inhibitor (GSK3i) induces compaction of synovial fibroblasts within the tissue resembling synovial lining in patient-derived organoids (Fig 1A). The effect of GSK3i on compaction of synovial lining was recapitulated synovial micromass where fibroblasts exposed to GSK3i formed a dense, compacted cellular network (Fig 1B). To determine the transcriptional changes induced by GSK3i, we performed scRNAseq and found GSK3i induced expression of lining fibroblast markers podoplanin (PDPN) and cadherin-23 (CDH23)5, consistent with a switch to a lining-like fibroblast phenotype (Fig 1C). The effect of GSK3i on lining fibroblast differentiation and compaction phenotype is reversible, as removal of GSK3i treatment induced a loss of lining fibroblast compaction (Fig 2A) and reduction in expression of lining fibroblast markers (Fig 2B), indicating that at a morphological and transcriptional level, fibroblast differentiation is reversible and active signaling is necessary to maintain a lining-like phenotype.
Conclusion: Our studies show that GSK3i induces a phenotypic and transcriptional switch towards synovial lining-like fibroblasts. Further investigations are underway to identify transcription factors necessary for lining fibroblast differentiation. Identification of transcriptional regulators will shed light on the molecular mechanism behind fibroblast heterogeneity and provide therapeutic targets for restoring synovial lining function in inflammatory arthritis.
- Bartok, B. & Firestein, G. S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255 (2010).
- Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
- Zhang, F. et al. Defining Inflammatory Cell States in Rheumatoid Arthritis Joint Synovial Tissues by Integrating Single-cell Transcriptomics and Mass Cytometry. doi:10.1101/351130.
- Wei, K. et al. Notch signaling drives synovial fibroblast identity and arthritis pathology. Nature 582, 259–264 (2020).
- Zhang, F. et al. Cellular deconstruction of inflamed synovium defines diverse inflammatory phenotypes in rheumatoid arthritis. bioRxiv 2022.02.25.481990 (2022) doi:10.1101/2022.02.25.481990.
To cite this abstract in AMA style:
Presti S, Watts G, Zhu Z, Bhamidipati K, Case J, Li Y, Bowman T, Kongthong S, Korsunsky i, Brenner M, Wei K. Patient-derived Organoids Reveal Transcriptional Regulation of Synovial Lining Fibroblast Differentiation [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/patient-derived-organoids-reveal-transcriptional-regulation-of-synovial-lining-fibroblast-differentiation/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-derived-organoids-reveal-transcriptional-regulation-of-synovial-lining-fibroblast-differentiation/