Session Information
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Prior work demonstrates that macrophages (MØs) from patients with Systemic Sclerosis (SSc) produce fibrotic and pro-inflammatory mediators and that cocultured MØs and SSc fibroblasts engage in reciprocal activation. However, the factors responsible for pro-fibrotic MØ activation in SSc are unknown. Given the proximity of MØs and fibroblasts in SSc patient skin, we hypothesize that fibroblast-derived mediators contribute to SSc MØ activation. In this study, we identify SSc dermal fibroblast-derived exosomes as inducers of MØ activation and demonstrate that exosome-activated MØs stimulate fibroblast production of inflammatory cytokines and extracellular matrix (ECM) components (Figure 1).
Methods: Fibroblasts were isolated from skin biopsies obtained from 9 SSc patients or 7 healthy age and gender-matched control subjects following written informed consent. CD14+ monocytes were purified from PBMCs obtained from whole blood of 7 healthy consented donors. Dermal fibroblasts were cultured with 5 ng/ml TGF-beta in media supplemented with exosome-depleted FBS for 72 hours prior to exosome isolation from cell supernatants. Exosomes were quantified using NanoSight and exosome purity was assessed by immunoblot for canonical markers. Monocytes were differentiated into MØs by incubation with M-CSF for 5 days and then were cultured with exosomes from control or SSc fibroblasts for an additional 48 hours. MØs were immunophenotyped using flow cytometry, qRT-PCR and multiplex. For mutual activation studies, exosome-activated MØs were co-cultured with SSc fibroblasts using Transwells.
Results: MØs activated with dermal fibroblast-derived exosomes from SSc patients upregulate surface expression of CD163, CD206, and MHC Class II compared with MØs incubated with healthy control fibroblasts (Figure 2). This pattern of expression is consistent with the previously identified surface marker profile established for human SSc MØs. In addition, SSc fibroblast-derived exosomes elicit elevated levels of secreted IL-6, IL-10, IL-12p40, and TNF from MØs (Figure 3). Exosome cargo from SSc and healthy control fibroblasts was sequenced and differential expression of microRNAs (miRs) was noted between the cell types, which may account for the enhanced inflammatory activation of SSc MØs. Co-culture studies demonstrate that exosome-stimulated MØs and SSc fibroblasts engage in reciprocal activation, as production of collagen and fibronectin is significantly increased in fibroblasts receiving signals from SSc exosome-stimulated MØs.
Conclusion: In this work, we demonstrate for the first time that human SSc dermal fibroblasts can mediate MØ activation through exosomes. Consistent with prior studies, we show that MØs express surface markers and release mediators associated with both alternative and inflammatory MØ activation. Our findings suggest that MØs and fibroblasts engage in cross-talk in SSc skin that results in mutual activation, inflammation, and ECM deposition. Collectively, these studies implicate MØs and fibroblasts as cooperative mediators of fibrosis in SSc and suggest dual therapeutic targeting of these cell types may provide maximal benefit in ameliorating disease in SSc patients.
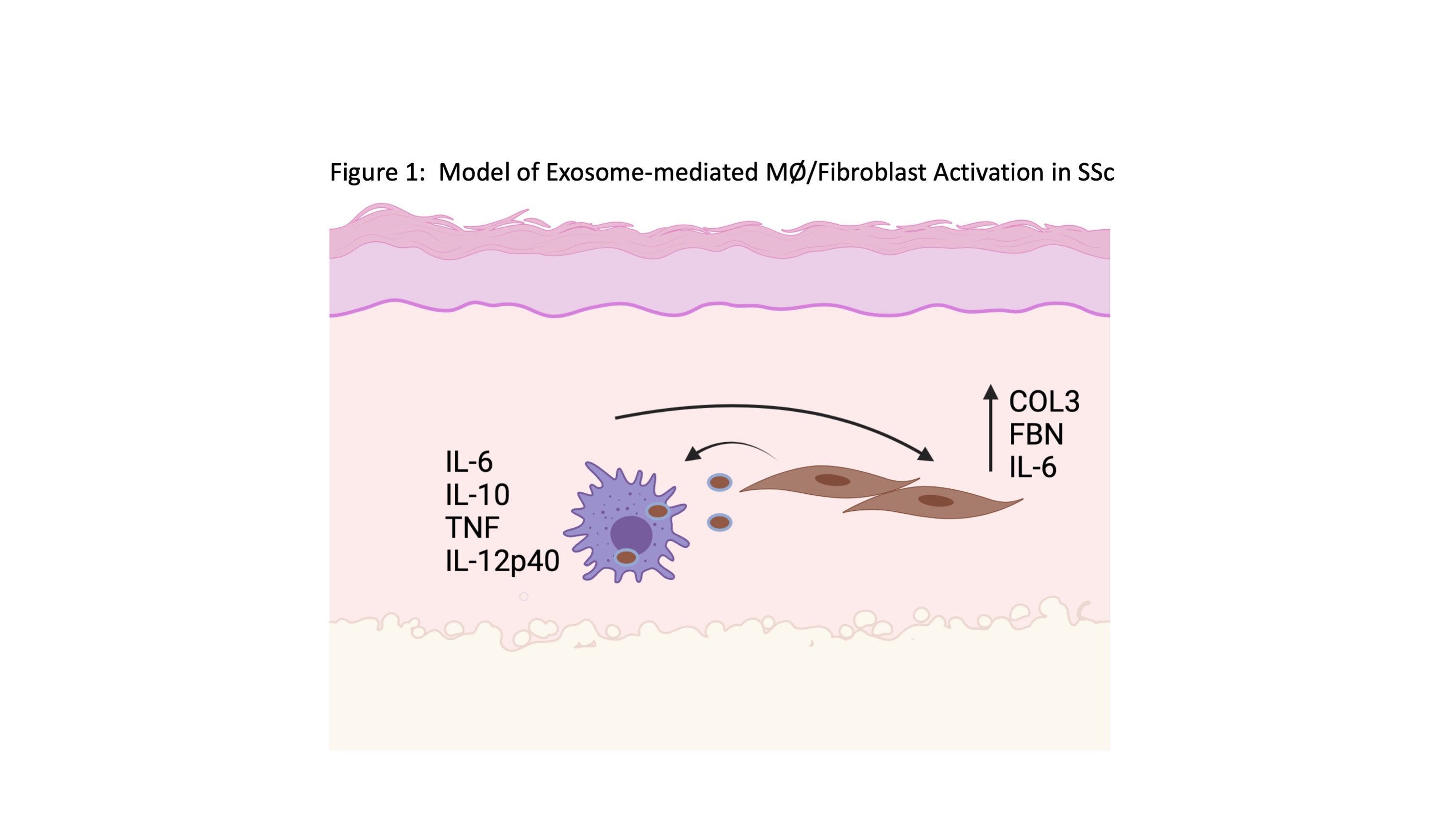 Figure 1: Model of SSc Fibroblast-derived Exosome-mediated Induction of MØ Activation in SSc. In our model, dermal fibroblasts from SSc patients release exosomes containing miR cargo that are internalized by MØs. Uptake of SSc fibroblast-derived exosomes induces pro-inflammatory and pro-fibrotic MØ activation, resulting in release of cytokines and fibrotic mediators by MØs that further stimulate SSc fibroblast activation. Cooperative activation results in dermal inflammation and increased ECM deposition.
Figure 1: Model of SSc Fibroblast-derived Exosome-mediated Induction of MØ Activation in SSc. In our model, dermal fibroblasts from SSc patients release exosomes containing miR cargo that are internalized by MØs. Uptake of SSc fibroblast-derived exosomes induces pro-inflammatory and pro-fibrotic MØ activation, resulting in release of cytokines and fibrotic mediators by MØs that further stimulate SSc fibroblast activation. Cooperative activation results in dermal inflammation and increased ECM deposition.
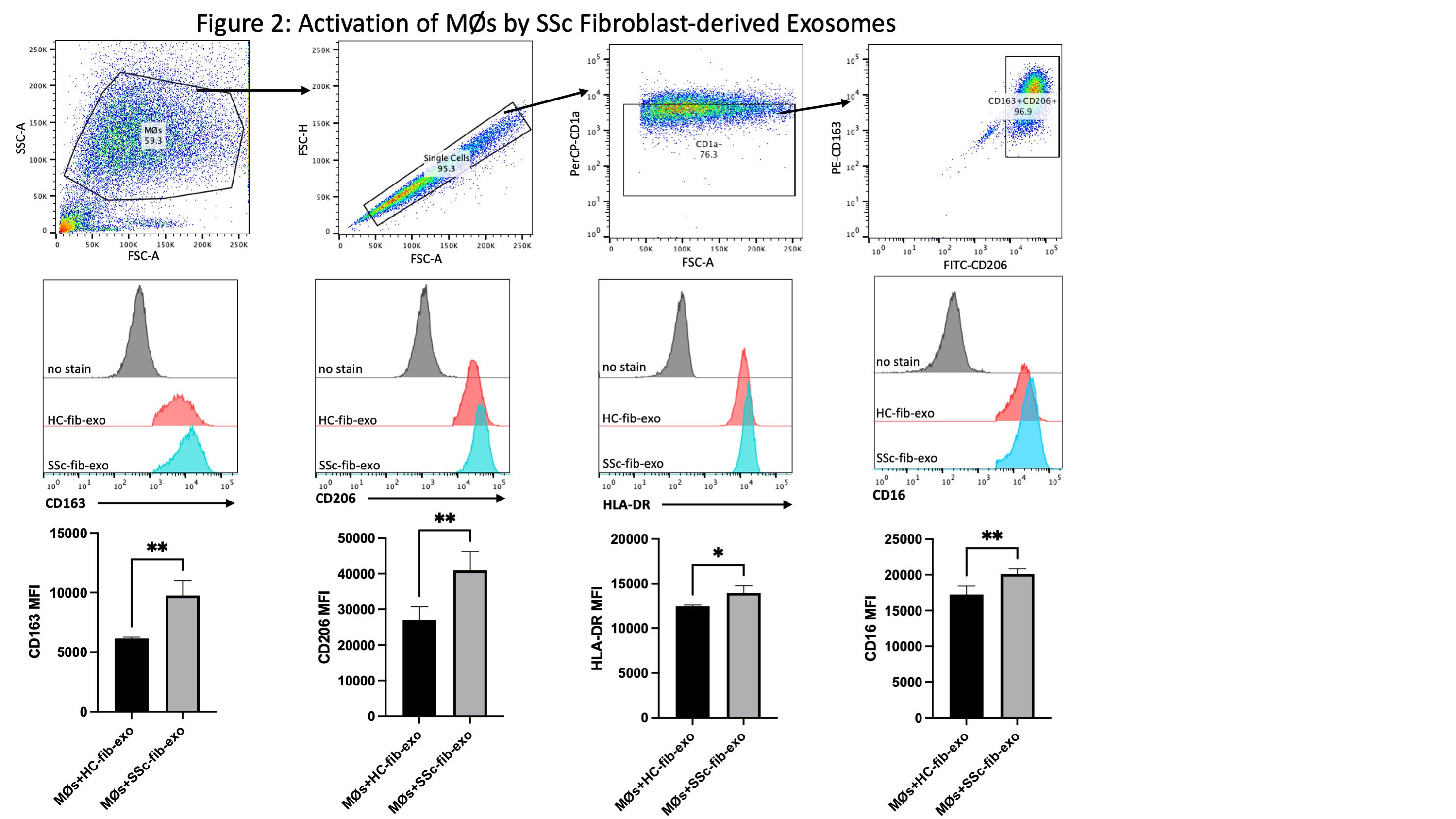 Figure 2: Induction of Surface Marker Expression on MØs by SSc Fibroblast-derived Exosomes. MØs were incubated with exosomes released from TGF-beta-stimulated SSc dermal or healthy control fibroblasts for 48 hours. Exosome uptake was verified in MØs using immunofluorescence, and activated MØs were immunophenotyped using flow cytometry. As demonstrated, internalization of SSc fibroblast-derived exosomes upregulated surface marker expression of CD163, CD206, and MHC Class II, which were previously identified as characteristic of pro-fibrotic SSc MØs.
Figure 2: Induction of Surface Marker Expression on MØs by SSc Fibroblast-derived Exosomes. MØs were incubated with exosomes released from TGF-beta-stimulated SSc dermal or healthy control fibroblasts for 48 hours. Exosome uptake was verified in MØs using immunofluorescence, and activated MØs were immunophenotyped using flow cytometry. As demonstrated, internalization of SSc fibroblast-derived exosomes upregulated surface marker expression of CD163, CD206, and MHC Class II, which were previously identified as characteristic of pro-fibrotic SSc MØs.
 Figure 3: Pro-inflammatory and Pro-fibrotic Cytokines Are Induced in MØs Activated with SSc-Fibroblast-derived Exosomes. Exosomes were isolated from the supernatant of TGF-beta stimulated SSc or healthy control fibroblasts and cultured with MØs for 48 hours. Exosome uptake was confirmed using immunofluorescence and MØ surface phenotype was monitored by flow cytometry. Supernatants were collected from activated MØs and analyzed using multiplex analysis.
Figure 3: Pro-inflammatory and Pro-fibrotic Cytokines Are Induced in MØs Activated with SSc-Fibroblast-derived Exosomes. Exosomes were isolated from the supernatant of TGF-beta stimulated SSc or healthy control fibroblasts and cultured with MØs for 48 hours. Exosome uptake was confirmed using immunofluorescence and MØ surface phenotype was monitored by flow cytometry. Supernatants were collected from activated MØs and analyzed using multiplex analysis.
To cite this abstract in AMA style:
Bhandari R, Yang H, Kosarek N, Whitfield M, Pioli P. Exosomes Mediate a Cooperative Mechanism of Macrophage/Fibroblast Activation in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/exosomes-mediate-a-cooperative-mechanism-of-macrophage-fibroblast-activation-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exosomes-mediate-a-cooperative-mechanism-of-macrophage-fibroblast-activation-in-systemic-sclerosis/
