Session Information
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: The use of Immune Checkpoint Inhibitors (ICIs) is limited by the induction of immune-related adverse events. CD6 is expressed by most T lymphocytes and a subset of natural killer (NK) cells, and engages the ligands CD166/ALCAM and CD318. Interrupting CD6 interaction with its ligands using UMCD6 (anti-CD6) reverses autoimmunity in mouse models of rheumatoid arthritis, multiple sclerosis and uveitis, due to suppression of differentiation of effector Th1 and Th17 cells. Recently, we have demonstrated that UMCD6 directly activates CD8+T and NK cells, enhancing these cells to kill breast, lung, and prostate cancer lines, even more robustly than ICIs directed to the PD-1/PD-1L pathway. We now explore the mechanisms by which UMCD6 activates NK cells while controlling the differentiation of CD4 cells.
Methods: RNAseq was used to study the molecular changes occurring during NK activation by CD6 blockade. Data analysis was conducted using DESeq2 software, and genes showing a Padjusted ≤0.05 were considered significantly differentially expressed.
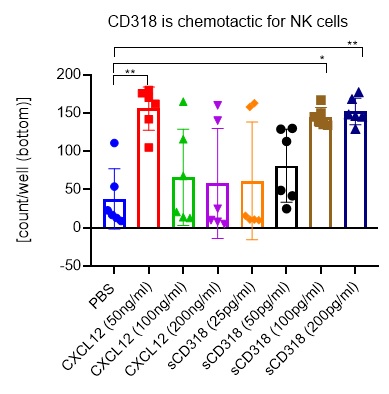
Chemotaxis of NK cells was assessed by the number of isolated human NK cells using an IncuCyte® Chemotaxis System assay. One-way ANOVA was used to compare groups. Significance was defined as p< 0.05.
To identify NK structures that are involved with the functional pathway that is triggered by UMCD6, we generated monoclonal antibodies from Balb/C mice immunized with NK-92 cells.
Results: Our RNAseq data from UMCD6-treated NK cells demonstrate extensive changes in gene expression induced by UMCD6. Expression of 180 genes was altered significantly. These genes included: i) activating NK receptors (e.g., Hcst [DAP10], and CD244 [2B4]), and ii) genes whose protein products are important in the immunoregulatory signaling pathways PI3K, AKT, and mTOR.
NK cells migrate in response to sCD318 with a peak response at 200 pg/ml (p < 0.01). This concentration of sCD318 is detectable in supernatants of cancer cell lines that we have used as targets for UMCD6-stimulated lymphocytes. NK cells also migrate toward stromal cell-derived factor (SDF)-1α (CXCL12) at a concentration of 25 ng/ml (p < 0.01), consistent with studies of NK chemotaxis to CXCL12 into sites of inflammatory responses or malignancies.
Hybridoma supernatants from Balb/C mice immunized with NK-92 cells have been screened to select antibodies whose corresponding surface structures are up-or down-regulated by UMCD6, co-internalize with UMCD6, and/or inhibit or augment target cell killing by NK cells. Thus far, 10 hybridomas have been selected based on informative positive results from this screening process.
Conclusion: The profound changes in gene expression induced by UMCD6 (anti-CD6) are consistent with our previous studies showing UMCD6 to concurrently control autoimmunity through effects on CD4+ lymphocyte differentiation while enhancing killing of cancer cells through activation of CD8+ and NK cells. The chemotactic properties of sCD318, a ligand of CD6, toward NK cells also demonstrates the importance of CD6 in migration of cytolytic lymphocytes into the tumor microenvironment. Altogether, these data point to a potential new approach to cancer immunotherapy that would suppress rather than instigate autoimmunity.
To cite this abstract in AMA style:
Gurrea-Rubio M, Ruth J, Wu Q, Tsou E, Campbell P, Randon P, Amin M, Singer N, Lin F, Fox D. The Mechanistic Basis of anti-CD6 as a Novel Form for the Treatment of Autoimmune Diseases and Cancer [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-mechanistic-basis-of-anti-cd6-as-a-novel-form-for-the-treatment-of-autoimmune-diseases-and-cancer/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-mechanistic-basis-of-anti-cd6-as-a-novel-form-for-the-treatment-of-autoimmune-diseases-and-cancer/