Session Information
Date: Saturday, November 6, 2021
Title: Sjögren's Syndrome – Basic & Clinical Science Poster (0296–0322)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: In a previous open-label phase II study, we showed that abatacept treatment might inhibit local formation of autoreactive memory B cells in parotid glands of primary Sjögren’s syndrome (pSS) patients by affecting germinal center (GC) formation, a process dependent on co-stimulation by activated follicular-helper-T-cells1. However, limitations of that study were small number of patients, restriction to parotid gland tissue and lack of a placebo group. Therefore the aim of this study was 1) assess the effect of abatacept in pSS patients based on analysis of parotid and labial gland biopsies, and 2) assess the prognostic value of histological characteristics of salivary glandular tissue with regard to responsiveness to abatacept treatment using the newly developed Composite of Relevant Endpoints for Sjogren’s Syndrome (CRESS)2.
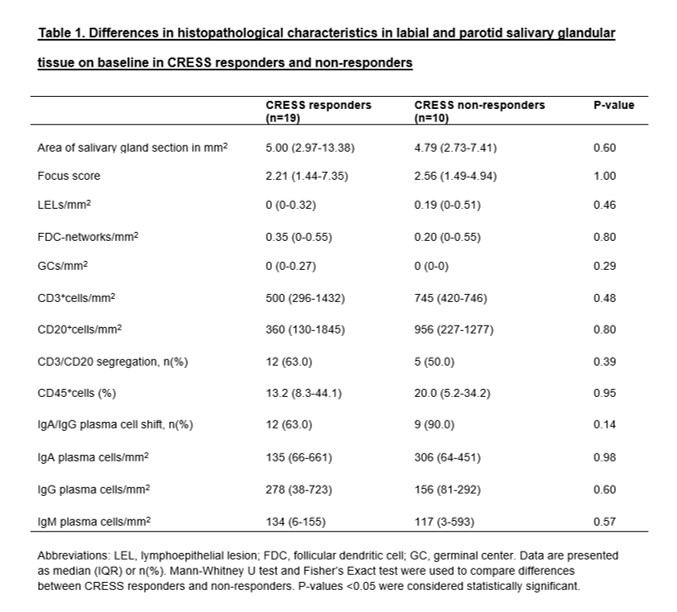
Methods: Patients from the Active Sjögren Abatacept Pilot (ASAP II, and ASAP III) and the international (IM101603) trial3,4 were combined in whom a labial (n=13) or parotid (n=30) salivary gland biopsy was obtained at baseline and after 24 weeks of treatment. Patients received either abatacept (n=27) or placebo (n=16) treatment. After salivary gland biopsies were processed, sections were stained with H&E and immunohistochemically for CD3, CD20, CD45, CD21, Bcl6, IgA/IgG, IgA, IgG and IgM. All sections were evaluated for focus score (FS), GCs, number of lymphoepithelial lesions (LELs) per mm2 and their maximal severity, presence of B/T-cell segregation within a focus, follicular dendritic cell (FDC) networks and presence of an IgA/IgG shift. Digital image analysis was used to assess the relative amount of CD45+lymphocytic infiltrates and the numbers of CD3+T cells, CD20+B cells and plasma cell density per mm2. Histopathological data at baseline were compared between CRESS responders (n=19) (response to ≥3 of the 5 items) and non-responders (n=10) to abatacept treatment2.
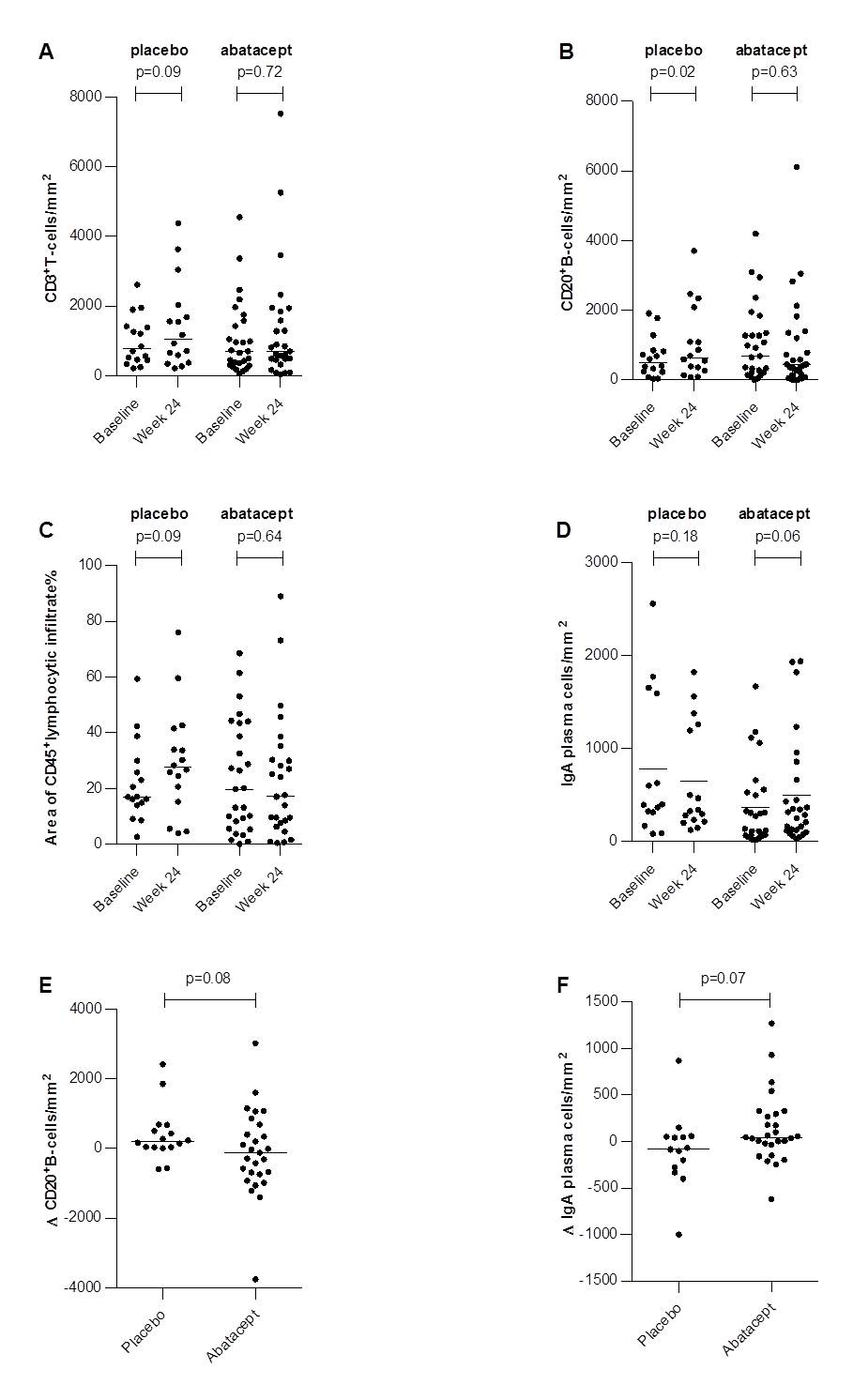
Results: In parotid and labial glandular sections, the number of CD20+B cells/mm2 increased significantly in placebo-treated patients (p=0.02), while counts remained stable in patients treated with abatacept. A similar trend was observed in the placebo group for CD3+T cells (p=0.09) and the relative area of CD45+infiltrate (p=0.09). The number of IgA plasma cells/mm2 increased after 24 weeks of abatacept treatment (p=0.06) (figure 1). No significant decrease in GCs/mm2 was observed in abatacept-treated pSS patients. Abatacept did not reduce FS, LELs/mm2, presence of B/T-cell segregation, presence of IgA/IgG shift, nor number of IgG and IgM plasma cells/mm2. No significant differences in histopathological parameters were found between CRESS responders and non-responders (table 1).
Conclusion: Abatacept potentially inhibits further progression of salivary gland inflammation by preventing further increase in T cell dependent B cell hyperactivity. Salivary gland histopathology could not predict responsiveness to abatacept in pSS patients.
References
1Haacke et al. Clin Exp Rheumatol. 2017;35(2):317-320
2Arends et al. Lancet Rheumatol. Online First: 2021. doi: 10.1016/S2665-9913(21)00122-3
3Meiners et al. Ann Rheum Dis. 2014;73(7):1393–96
4van Nimwegen et al. Lancet Rheumatol. 2020;9913(19):1-11
Wilcoxon signed-rank test and McNemar’s test were used to compare differences over time within groups. Mann-Whitney U test and Fisher’s Exact test were used to compare differences between the abatacept and placebo groups. P-values <0.05 were considered statistically significant.
To cite this abstract in AMA style:
Nakshbandi U, de Wolff L, Kroese F, Liefers S, Ray N, Verstappen G, Spijkervet F, Nys M, Vissink A, Wong R, van der vegt B, Bootsma H. Histopathological Changes in Parotid and Labial Salivary Gland Tissue in Primary Sjögren’s Syndrome Patients After Abatacept Treatment [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/histopathological-changes-in-parotid-and-labial-salivary-gland-tissue-in-primary-sjogrens-syndrome-patients-after-abatacept-treatment/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/histopathological-changes-in-parotid-and-labial-salivary-gland-tissue-in-primary-sjogrens-syndrome-patients-after-abatacept-treatment/