Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: The Pediatric Rheumatology Care and Outcomes Improvement Network (PR-COIN) is a learning network to support pediatric rheumatology centers in improving care delivery and patient outcomes. This growing network, now 21 centers, was established in 2011 and has tracked outcome and process measures over time. PR-COIN recently modified its quality measure (QM) set that coincided with a registry migration of 5,905 patients to a new platform to support optimal data reporting. This report highlights the network’s new QMs and provides some performance on these measures from a large JIA cohort enrolled in the network registry.
Methods: The network’s initial process measures were based on a published set of QMs for JIA that was developed utilizing an evidence-based approach and consensus methodology. Outcome measures were created using published, validated JIA outcome assessments. Taking into consideration 3 main measure attributes – clinical health importance, scientific validity, and feasibility – in addition to new best practices, PR-COIN modified the QM set in 2019 with parent input and developed operational definitions. QMs were tested using patient data for validation. Network teams enter and/or auto-upload patient data into the PR-COIN registry to populate the measures, and statistical process control charts are utilized to display monthly performance.
Results: PR-COIN’s QM set consists of 20 measures, including 16 clinical and 4 data quality measures (Table 1). Outcome measures for disease control include evaluation of the clinical Juvenile Arthritis Disease Activity Score (cJADAS10), clinically inactive disease, the active joint count, and the time to inactive disease. Patient-reported outcomes are comprised of optimal physical function, low or no pain, low patient global assessment of overall well-being, and low pain interference. Treat to Target interventions, including setting a treatment target and use of clinical decision support, will be tracked as process measures in addition to the use of self-management support and appropriate medication safety monitoring. A new balancing measure regarding infection-related hospitalizations rounds out the QM set. Figure 1 is an example run chart of a disease control outcome measure – mean disease activity score by cJADAS10 (scale 0-30) of all JIA patients – demonstrating the aggregate performance of 8 PR-COIN sites. This cumulative measure has been stable at 2.5 over the last 5 months. Figure 2 is an example run chart using collaborative data of a patient-reported quality of life outcome measure, the mean patient global assessment of well-being (scale 0-10). This score has been around 1.26 for the last several months.
Conclusion: PR-COIN has enhanced its comprehensive and varied QM set for the care of patients with JIA. Having clinically important measures that are tested for feasibility on a large cohort of patients with JIA can maximize quality improvement efforts to optimize care delivery.
 Table 1. PR-COIN Quality Measures for Juvenile Idiopathic Arthritis.
Table 1. PR-COIN Quality Measures for Juvenile Idiopathic Arthritis.
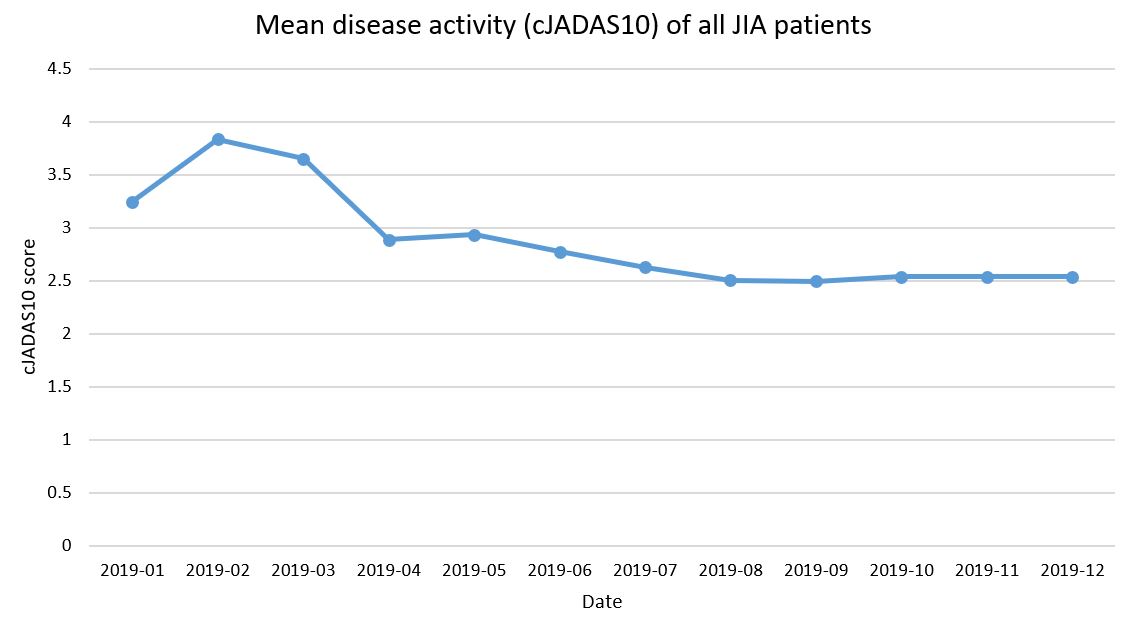 Figure 1. Mean disease activity score by cJADAS10 of JIA patients in PR-COIN.
Figure 1. Mean disease activity score by cJADAS10 of JIA patients in PR-COIN.
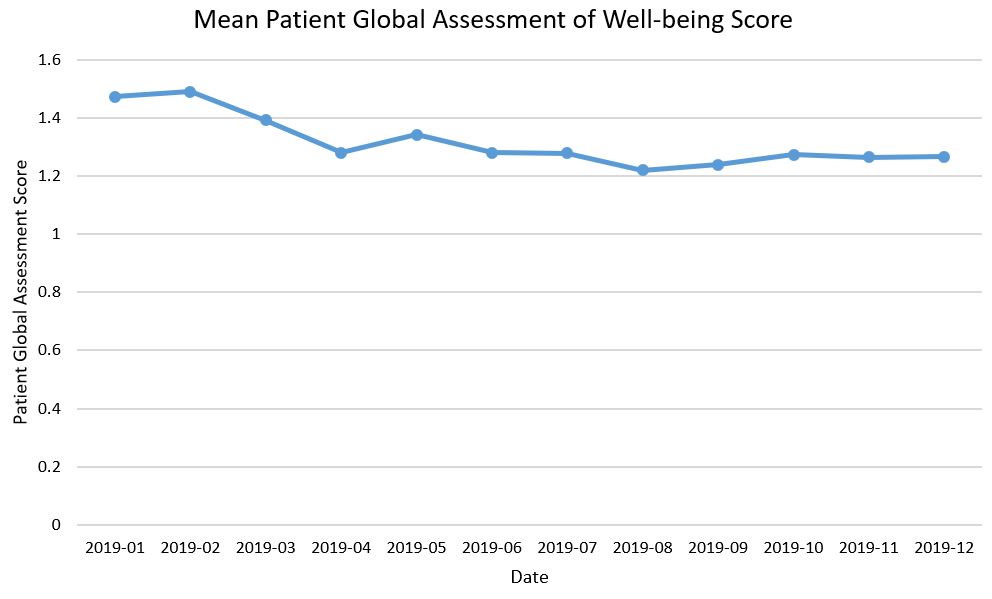 Figure 2. Mean patient global assessment of well-being score for JIA patients in PR-COIN.
Figure 2. Mean patient global assessment of well-being score for JIA patients in PR-COIN.
To cite this abstract in AMA style:
Harris J, Morgan E, Vora S, Gilbert M, Yildirim-Toruner C, Griffin N, Ferraro K, Loos S, Qiu T, Paul A, Burnham J, Batthish M, Gottlieb B, Bullock D, Hazen M, Laxer R, Lee T, Mannion M, Olson J, Pan N, Shishov M, Spencer C, Weiss J, Bingham C. New Juvenile Idiopathic Arthritis Quality Measure Set for the Pediatric Rheumatology Care and Outcomes Improvement Network [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/new-juvenile-idiopathic-arthritis-quality-measure-set-for-the-pediatric-rheumatology-care-and-outcomes-improvement-network-2/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/new-juvenile-idiopathic-arthritis-quality-measure-set-for-the-pediatric-rheumatology-care-and-outcomes-improvement-network-2/
