Session Information
Date: Saturday, November 7, 2020
Title: Innate Immunity (0945–0949)
Session Type: Abstract Session
Session Time: 10:00AM-10:50AM
Background/Purpose: Monocytes orchestrate immune responses that protect against microbes but can also drive pathological inflammation and autoimmune disease. Monocytes are thought to be activated primarily by cytokines or by microbial molecules, but they express a broad array of receptors allowing them to integrate responses from the environment. We hypothesize that monocytes may be activated by a first, or primary signal, and that a second, booster stimulus can then drive a pathological super-activated state. To address this hypothesis, we analyzed high dimensional transcriptomic data for monocytes from diseased human tissue to identify receptors that might serve as super-activators of these cells, and identified SLAMF7, a receptor that regulates leukocyte activation.
Methods: We analyzed bulk RNA-sequencing (RNA-seq) data from Phase I of the Accelerating Medicines Partnership to identify genes upregulated in monocytes from rheumatoid arthritis synovial tissue compared to osteoarthritis synovial tissue. We used flow cytometry to quantify this receptor on monocytes and sorted monocytes with high versus low SLAMF7 expression for RNA-seq. We used an activating antibody or recombinant SLAMF7 protein to stimulate monocytes in vitro and performed ELISA to measured cytokine release and quantitative real-time PCR to quantify gene expression. We also collected RNA-seq data from monocytes after stimulation and identified upregulated genes as the “SLAMF7 Activation Signature.” We than analyzed publicly available single cell RNA-seq data from individuals with RA, Crohn’s disease and COVID-19 lung infection with the “SLAMF7 Activation Signature.”
Results: Through differential gene expression analysis we identified SLAMF7 as a receptor that is profoundly upregulated in monocytes from inflamed synovial tissues (fig 1). We identified an interferon signature in SLAMF7-high monocytes, and additional in vitro stimulation experiments implicated IFN-γ as a key regulator of SLAMF7 expression. Engaging SLAMF7 with an antibody or recombinant protein on monocytes drove profound production of inflammatory cytokines TNF-α, IL-6, and IL-1β, and chemokines CCL3, CXCL1, and CXCL8. We performed transcriptome-wide analysis on these stimulated monocytes to generate a “SLAMF7 Activation Signature”. We observed a striking overlap between our experimental signature and the transcriptional signature identified in infiltrating monocytes from RA synovial tissue, bronchoalveolar lavage cells of patients with COVID-19, as well as from ileal tissue of patients with Crohn’s disease.
Conclusion: SLAMF7 is a receptor that is massively upregulated on monocytes from inflamed tissues. IFN-γ drives monocytes to express high levels of SLAMF7 but does not drive complete monocyte activation on its own. A second booster activation signal through engagement of SLAMF7 then triggers profound super-activation, resulting in monocyte production of high levels of inflammatory effector molecules. We implicate this pathway as a major contributor to pathologic inflammation in human diseases. Therapies targeting different steps in monocyte super-activation could have potential for treating diverse human diseases.
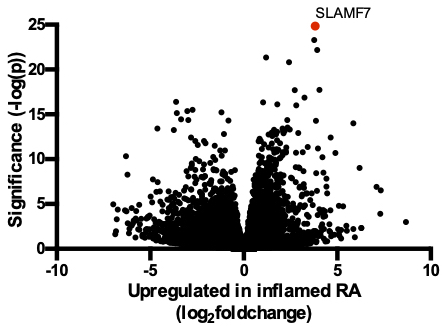 Figure 1. Upregulation of SLAMF7 in inflamed synovial tissues. Differentially expressed genes in synovial monocytes from patients with inflamed RA (n=11) compared to OA (n=10) from AMP Phase 1 bulk RNA-sequencing are shown, with SLAMF7 labeled in red.
Figure 1. Upregulation of SLAMF7 in inflamed synovial tissues. Differentially expressed genes in synovial monocytes from patients with inflamed RA (n=11) compared to OA (n=10) from AMP Phase 1 bulk RNA-sequencing are shown, with SLAMF7 labeled in red.
To cite this abstract in AMA style:
Simmons D, Nguyen H, Gomez-Rivas E, Jeong Y, Apruzzese W, Kim E, Brenner M. SLAMF7 Engagement Drives Monocyte Super-Activation in Acute and Chronic Inflammation [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/slamf7-engagement-drives-monocyte-super-activation-in-acute-and-chronic-inflammation/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/slamf7-engagement-drives-monocyte-super-activation-in-acute-and-chronic-inflammation/
