Session Information
Date: Monday, November 11, 2019
Title: RA – Animal Models Poster
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: We previously identified that under homeostatic conditions Flip was necessary for macrophage (MΦ) survival. The mechanisms responsible for the differentiation of monocyte-derived MΦs into anti-inflammatory tissue-resident MΦs (TRMs), under chronic inflammatory conditions, is not known. Earlier studies demonstrated that under homeostatic conditions, efferocytosis is important in maintaining MΦ tissue residency. The purpose of this study was to define synovial TRMs and the role of Flip in MΦ differentiation during chronic inflammation.
Methods: The HUPO arthritis model was established employing mice with Flip depleted in CD11c+ cells. HUPO arthritis, which begins spontaneously after 4 weeks of age, was evaluated by clinical scoring and histology. Synovial tissue MΦ subsets were defined by flow cytometry and proliferation by BrdU incorporation. Transcriptional profiles for each subset was documented by RNAseq. Monocyte-derived MΦs in joint tissue were identified following bone marrow reconstitution and parabiosis.
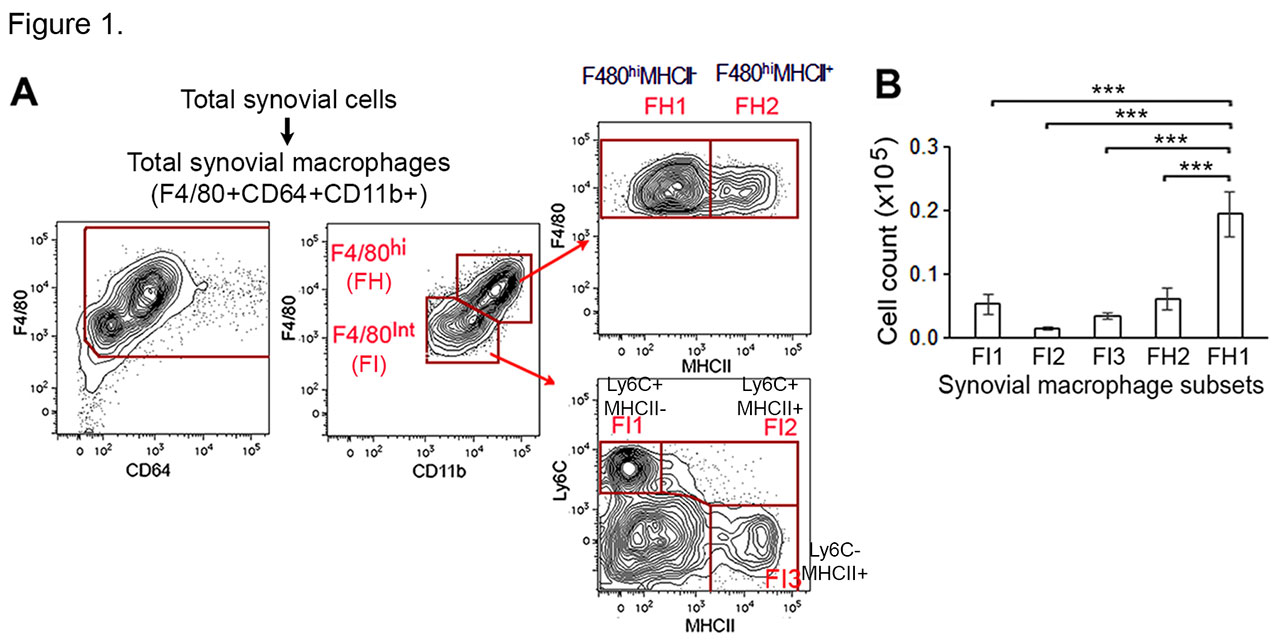
Results: We identified the F4/80hiMHCII–Ly6C– subset (FH1) as the population of TRMs, dominant among the 5 subsets of synovial MΦs during homeostasis (Fig 1). Genes that are upregulated in the TRMs have tissue-specific functions such as for MΦ maturation and maintenance of homeostasis. The wild type FH1 and FH2 (F4/80hi MHCII+Ly6C– ) subsets are maintained at a slow self-renewing rate and not replenished by circulating monocytes. The cflar (Flip) expression is high in F4/80hi MΦs, and targeted deletion of Flip in the Flipf/f CD11ccre HUPO mice results in a marked reduction of the FH1 population, while the FH2 subset is greatly increased. The percent of the FH1 subset inversely correlated with arthritis severity and granulocyte infiltration. Parabiosis experiments demonstrated both HUPO and control monocytes differentiated into FH2 MΦs in HUPO recipients, while only the control MΦs were able to differentiate into FH1 cells. This inability of HUPO monocytes to differentiate from FH2 to FH1 MΦs was not due to increased MΦ apoptosis. The reduced Flip did not result in increased apoptosis was associated with a reduction of pro-apoptotic, and an increase of anti-apoptotic signaling molecules. Further, the HUPO FH2 MΦs demonstrated a reduction of genes and their coding proteins such as Cfs1r (CD115), Tgfbr2, and Mrc1 (CD206), which are associated with tissue residency and efferocytosis. In addition, genes important for MΦ functions and phagocytic clearance of apoptotic cells, such as Timd4 and CD163 were reduced in the HUPO FH1 and FH2 subsets.
Conclusion: These observations suggest that under homeostatic conditions, efferocytosis maintains the dominance of the FH1 population as TRMs. In contrast, apoptosis is reduced, and all populations of MΦs, except FH1, are increased under HUPO chronic inflammatory conditions. FH2 MΦs deficient in Flip survive, but are not capable of differentiation into anti-inflammatory TRMs. These observations provide novel insights into the role of MΦs in the initiation and progression rheumatoid arthritis.
To cite this abstract in AMA style:
Huang Q, Doyle R, Chen S, Misharin A, Winter D, Pope R. The Role of Flip in Differentiation and Survival of Synovial Tissue Resident Macrophages [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-role-of-flip-in-differentiation-and-survival-of-synovial-tissue-resident-macrophages/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-role-of-flip-in-differentiation-and-survival-of-synovial-tissue-resident-macrophages/