Session Information
Date: Tuesday, November 14, 2023
Title: (1945–1972) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Polymyositis (PM) and dermatomyositis (DM) are chronic autoimmune diseases characterized by muscle inflammation and weakness. In a collagen-induced myositis mouse model, immunoproteasome inhibition has shown to significantly revert the decreased grip strength and reduce the histopathological score of inflamed striated muscles. Zetomipzomib (KZR-616) is a first-in-class selective inhibitor of the immunoproteasome. PRESIDIO (NCT04033926) was a Phase 2 randomized, double-blind, placebo-controlled crossover study evaluating the safety, tolerability, efficacy and PK/PD of zetomipzomib 45 mg subcutaneously (SC) weekly (QW) treatment in patients (pts) with active PM and DM with an open-label extension (OLE) to evaluate the long-term efficacy and safety up to 96 weeks (NCT04628936). Results from PRESIDIO and its OLE study are reported here.
Methods: Pts were randomized to initially receive zetomipzomib 45 mg (first two doses: 30 mg) or placebo SC QW; pts received zetomipzomib or placebo treatment over 16 weeks, followed by crossover to the other arm for an additional 16 weeks (total study duration of 32 weeks). Study entry criteria included adults with probable or definite PM or DM based on the 2017 EULAR/ACR Classification Criteria with confirmed active disease (MMT-8 score of 80-136 and two other abnormal core set measures). The primary endpoint was the mean change in the Total Improvement Score (TIS). Pts completing 32 weeks of the double-blind treatment period could enroll in the OLE study up to 96 weeks.
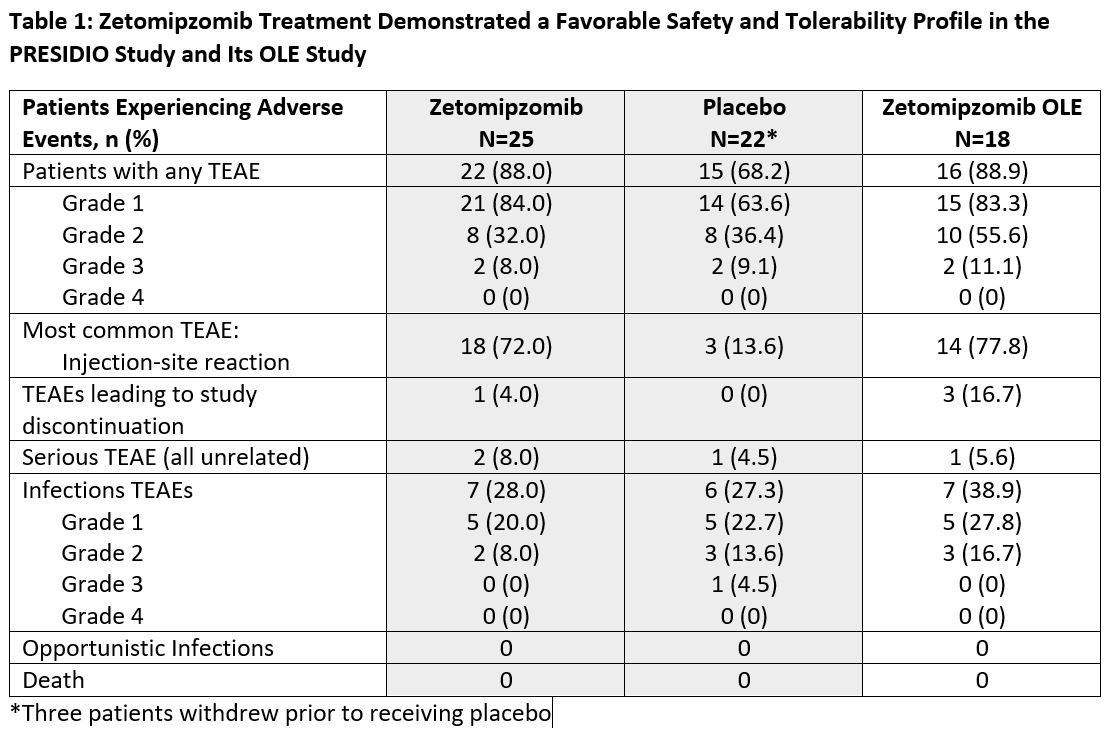
Results: 25 pts with active DM (n=13) and PM (n=12) enrolled and 20 pts completed through end-of-treatment; 18 opted into the OLE study (4 pts received < 24 weeks of zetomipzomib dosing, 4 pts received 24-48 weeks of dosing, and 10 pts received ≥48 weeks of zetomipzomib dosing [1 pt had 96 weeks of treatment]). Overall, pts saw clinically meaningful improvements in TIS, but zetomipzomib demonstrated no significant differentiation from placebo. At Week 16, the zetomipzomib group achieved a mean TIS of 25.5 versus 25.0 in the placebo group. Following cross-over at Week 32, the pts receiving zetomipzomib beginning at Week 16 achieved a mean TIS of 34.2 versus 34.1 in those receiving placebo beginning at Week 16. Zetomipzomib treated patients also showed improvements in other efficacy endpoints. In the OLE, mean TIS generally continued to improve with reduced symptoms and improvement in strength. Treatment-emergent adverse events (TEAE) were generally mild-to-moderate (Table 1). The most common TEAEs were injection site reactions (ISRs), which were generally transient and manageable. ISRs occurred more frequently with zetomipzomib than with placebo. There was no evidence of immunosuppression observed, as measured by preserved cell counts and lack of ≥ Grade 3 infection or opportunistic infections. There were no off-target or new adverse events observed with >1-year weekly administration in the OLE.
Conclusion: Zetomipzomib demonstrated a favorable safety and tolerability profile without signs of direct immunosuppression upon prolonged exposure. Zetomipzomib did not show differentiation from placebo, but individual patients with DM or PM experienced a clinically meaningful improvement in TIS.
To cite this abstract in AMA style:
Aggarwal R, Goyal N, Lam D, Ng A, Leff R, Ray K, Park E, Henig N. Zetomipzomib Demonstrates Favorable Long-term Safety and Tolerability Profile Without Signs of Immunosuppression: Results from the PRESIDIO Study and Its Open-label Extension Study in Patients with Dermatomyositis and Polymyositis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/zetomipzomib-demonstrates-favorable-long-term-safety-and-tolerability-profile-without-signs-of-immunosuppression-results-from-the-presidio-study-and-its-open-label-extension-study-in-patients-with-de/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/zetomipzomib-demonstrates-favorable-long-term-safety-and-tolerability-profile-without-signs-of-immunosuppression-results-from-the-presidio-study-and-its-open-label-extension-study-in-patients-with-de/