Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Measures & Measurement of Healthcare Quality (2615–2620)
Session Type: Abstract Session
Session Time: 4:00PM-4:15PM
Background/Purpose: Despite widespread recognition of persistent patient safety challenges in the U.S. health care system, the development of feasible and scalable solutions has lagged, particularly for ambulatory patients receiving immunosuppressive therapies. In this qualitative study we interviewed stakeholders across a diverse group of rheumatology practices to identify barriers and facilitators of high performance on four national patient safety measures, including screening for Hepatitis B and Tuberculosis prior to initiating immunosuppressive therapy, recommended Hydroxychloroquine dosing, and osteoporosis assessment for high-risk populations.
Methods: We identified 139 practices participating in the ACR’s RISE registry with above-average performance on ≥1 of the 4 patient safety measures for interviews. Since RISE practices are largely community-based, we also invited 6 academic (AMCs) and 5 Veterans Affairs (VA) medical centers. Rheumatologists and key staff members involved in patient safety measure workflows were invited to take part in virtual semi-structured interviews. Guided by the concepts of the Consolidated Framework for Implementation Research (CFIR), the recorded interviews were transcribed verbatim and analyzed thematically using deductive and inductive techniques to generate themes.
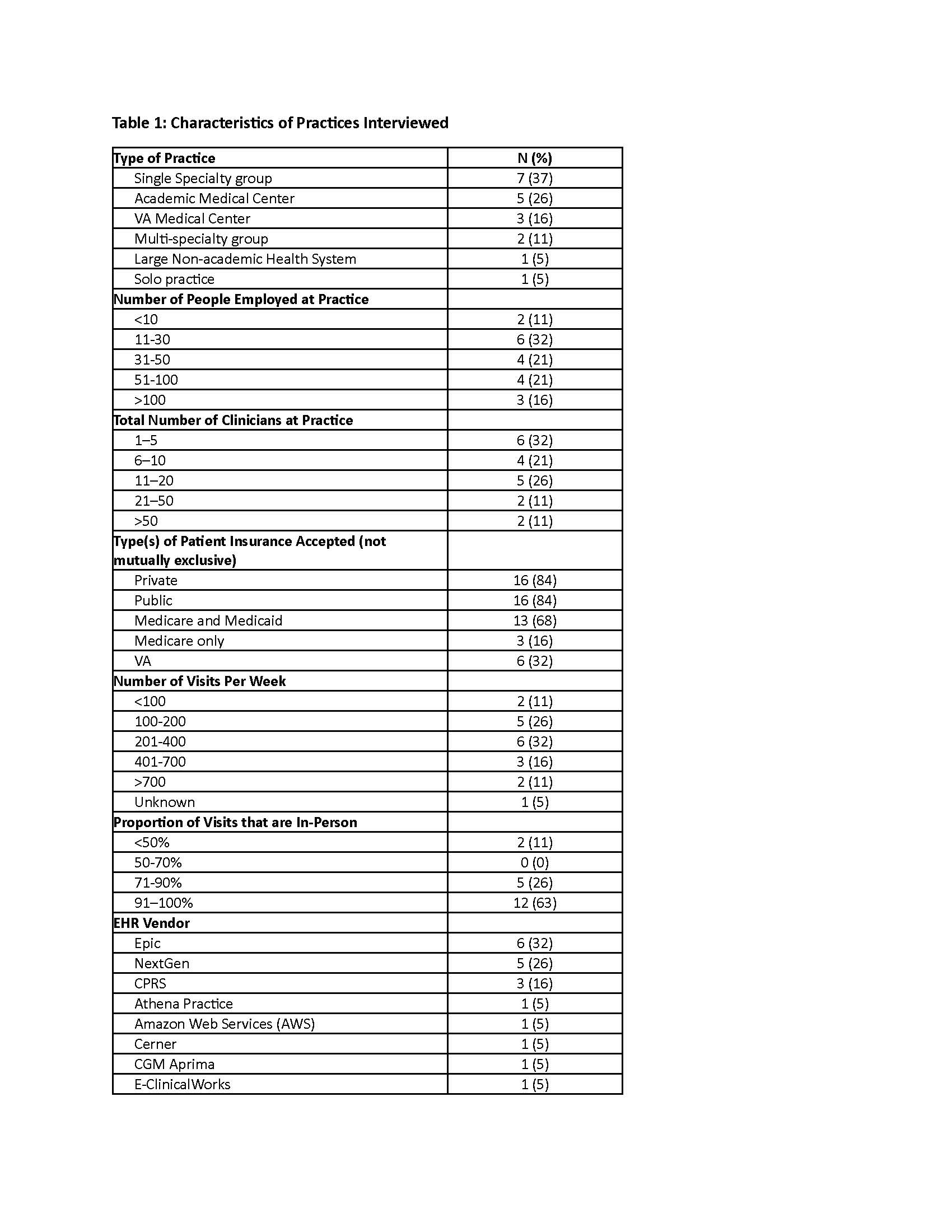
Results: We conducted 19 interviews with 21 participants across 11 RISE practices, 5 AMCs, and 3 VAs. Participants included 11 rheumatologists, 6 practice managers, 2 pharmacists, 1 nurse, and 1 quality and compliance manager (Table 1). Practices described several innovative workflows that supported high performance on quality measures. These included standardized screening protocols and safety monitoring tools, often incorporating EHR-based alerts, integrated screening data, performance dashboards, and customized EHR fields for documentation. High performance was commonly attributed to clinicians’ strong commitment to patient safety, ongoing staff training on clinical guidelines, and interdisciplinary collaboration, particularly with pharmacists (Table 2). Respondents also identified persistent challenges including inefficient workflows for repatriating external lab data, inconsistent or missing documentation of screening information in structured EHR fields, non-standardized workflows and logistical difficulties in tracking the implementation and performance of safety measures (Table 3).
Conclusion: This study provided critical insights into key barriers and facilitators affecting rheumatology practices’ performance on patient safety measures. Qualitative interviews revealed strategies associated with higher performance on safety measures, such as EHR-supported protocols and collaboration with pharmacists, but also highlighted challenges like inefficient workflows for data entry and data integration issues. These findings have laid the foundation for the development of the Rheum Safe Patient Safety Toolkit, currently in development. The toolkit will guide RISE practices in implementing patient safety measures and optimizing workflows for scalable and sustained improvement.
To cite this abstract in AMA style:
Nasrallah C, Wilson C, Hariz C, Hamblin A, Young C, Schmajuk g, Yazdany J. What Works? A Consolidated Framework for Implementation Research-Guided Exploration of Patient Safety in Rheumatology Practices [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/what-works-a-consolidated-framework-for-implementation-research-guided-exploration-of-patient-safety-in-rheumatology-practices/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-works-a-consolidated-framework-for-implementation-research-guided-exploration-of-patient-safety-in-rheumatology-practices/

.jpg)
.jpg)