Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: With the popularity of medication-assisted weight loss, the current study seeks to characterize the association of weight loss, disease activity, and patient reported outcomes (PROs) in patients with musculoskeletal and autoimmune disease using these therapies. We hypothesize that those experiencing weight loss would experience greater improvements in disease activity and PROs.
Methods: Participants were selected the FORWARD Databank. Adults are enrolled from community-based rheumatology practices across the US and are asked to complete questionnaires every 6 months. Participants were included in this study if they reported the use of a medication associated with weight loss from 1/1/2005-6/1/2023; all observations were included from this period, regardless of use of weight loss therapy at a given observation; observations before and after initiation of weight loss therapy were included. The primary condition was diagnosed by a treating rheumatologist; other data were self-reported. PROs included in this study include patient global assessment, pain scale, fatigue scale, polysymptomatic distress (PSD) questionnaire, health activity questionnaire (HAQ-2), short form survey 36 (SF-36), and patient activity score II (PAS-II, a composite including HAQ-2, patient global, and pain). Linear regression and linear models using generalized estimating equations were applied to assess the association with achieving ≥5% weight loss and changes in PROs over a 6-month period, clustering by patient and adjusting for the prior 6-month PRO, BMI, diabetes status, age, and sex. Testing for effect modification was performed to assess the impact of weight loss across BMI categories and primary diagnosis.
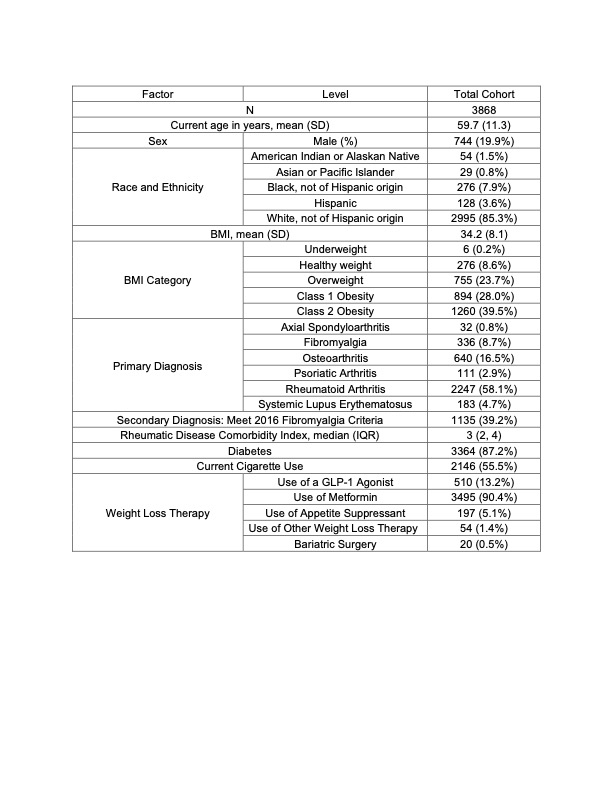
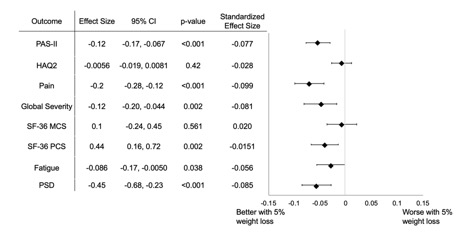
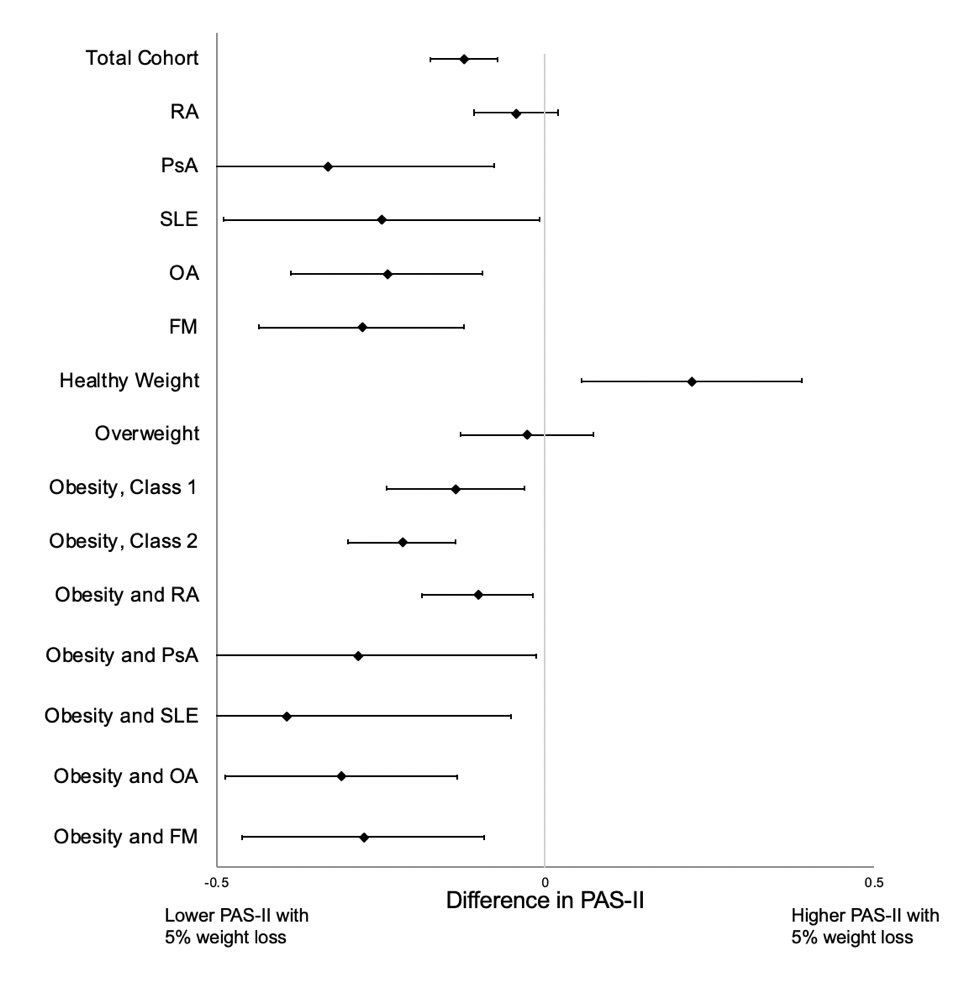
Results: In total, 3,868 participants were eligible for the current study, with 24,484 discrete observations where a six-month weight change could be calculated. 5% (1261) of observations occurred after initiation of a weight loss therapy, and 49% (12047) occurred with prevalent use. 58.1% of participants had a primary diagnosis of RA, 16.5% had OA, 8.7% FM, and 2.9% PsA (Table 1). 5% weight loss was reported in 10.5 % (2,603) of observations. In unadjusted and adjusted analysis, observations with ≥5% weight loss had statistically significant improvements in PAS-II, patient global assessment, pain, fatigue, polysymptomatic distress, and SF-36 physical component score (PCS) (PAS-II adjusted: Β -0.12, 95% CI -0.17, -0.067, p < 0.001, others in Fig. 1). In testing for effect modification, those who lost weight were more likely to have a change in PAS-II and PROs if they were obese with any diagnosis (Fig. 2). Weight loss was not associated with improvements in PAS-II or PROs among patients with a BMI < 25 or among all participants with RA.
Conclusion: Among those taking weight loss therapies, ≥5% weight loss was associated with statistically significant improvements in patient-reported disease activity and other quality of life PROs. Patients with obesity, regardless of underlying condition, were most likely to improve. These observations may inform randomized clinical trials in these conditions aimed to assess if medication-assisted weight loss can improve symptoms and other features of disease activity.
appetite suppressants include phentermine benzphetamine diethylpropion phendimetrazine; other weight loss therapies include: orlistat, naltrexone/buprenorphine, phentermine/topiramate
To cite this abstract in AMA style:
Riley T, Wipfler K, Wysham K, George M, Michaud K, Baker J. Weight Loss, Disease Activity, and Patient Reported Outcomes in Patients with Musculoskeletal and Autoimmune Diseases Taking Weight Loss Therapies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/weight-loss-disease-activity-and-patient-reported-outcomes-in-patients-with-musculoskeletal-and-autoimmune-diseases-taking-weight-loss-therapies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/weight-loss-disease-activity-and-patient-reported-outcomes-in-patients-with-musculoskeletal-and-autoimmune-diseases-taking-weight-loss-therapies/