Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Tocilizumab (TCZ), a biologic Disease Modifying Anti-Rheumatic Drug, elicits an immune-modulating effect by binding to the interleukin 6 receptor and inhibiting downstream signaling. Despite reported effectiveness in early rheumatoid arthritis (RA) patients, about a third do not reach sufficient response [1]. Previously, we performed baseline RNA sequencing (RNA-seq) in discovery and validation cohorts of early RA patients treated with TCZ [2]. Two significantly differentially expressed genes were identified in the discovery cohort and nine were identified in the validation cohort. In both cohorts, urotensin-2 (UTS2) gene expression was found to be significant between TCZ-responders and non-responders [2]. UTS2 (protein) has proposed function as a vasoconstrictor and was increased in synovial fluid in osteoarthritis [3]. To further establish UTS2 as a potential biomarker for the prediction of TCZ non-response in RA, we validated the mRNA expression of UTS2 in the validation cohort by qPCR.
Methods: Heparinized blood was collected at baseline from 96 therapy-naïve early RA patients enrolled in the U-Act Early trial (validation cohort NCT01034137) [1]. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation, followed by RNA isolation and cDNA synthesis. The mRNA expression of UTS2 was assessed by qPCR with β-glucuronidase (GUSB) as the reference gene. Patients were stratified as TCZ responders (R) and non-responders (NR) based on the low disease activity by either DAS28-ESR or cDAI (defined as DAS28-ESR < 3.2 or cDAI > 2.8 and ≤ 10) at 6 months. Two response criteria were used as TCZ use affects ESR and CRP [1]. Therapy responses were compared using Mann-Whitney U-test and p values of < 0.05 were considered statistically significant.
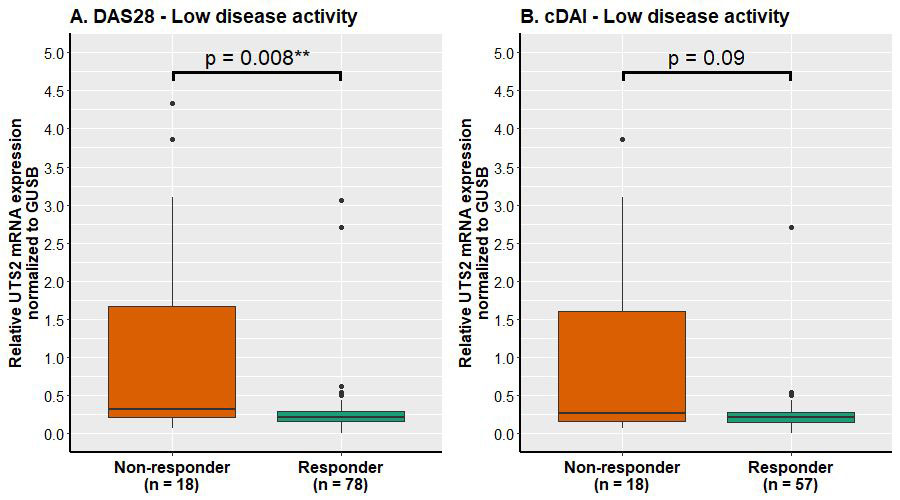
Results: Baseline mRNA expression of UTS2 from PBMCs was assessed in 96 therapy-naïve RA patients. Stratification with DAS28-ESR resulted in 18 NR and 78 R, cDAI resulted in 18 NR and 57 R (cDAI was available for 75 patients). The mean ± SD of relative mRNA expression of UTS2 was significantly higher (3.5-fold) in NR than R (based on DAS28ESR): 1.05±1.39 vs 0.30±0.44, respectively (p= 0.008, Figure 1A). The mean ± SD of relative mRNA expression of UTS2 3.6-fold higher in NR than R based on cDAI: 0.94 ±1.25 vs 0.26±0.35, respectively (p=0.09, Figure 1B).
Conclusion: UTS2 expression, originally identified as a candidate marker for TCZ non-response in RNA-seq analyses, was validated by qPCR in 96 baseline PBMC samples of early RA patients starting TCZ therapy. qPCR data verified RNA-seq data with significantly higher UTS2 mRNA expression in TCZ non-responders compared to responders for DAS28-ESR and significant trend for cDAI response criteria. The function of UTS2 in RA and in immune diseases [4] overall is poorly explored and warrants further investigation.
References
[1] JWJ Bijlsma et al, Lancet 2016;388:343–355; [2] I.B. Muller et al, Ann Rheum Dis 2021;80(Suppl 1):12; [3] B Gögebakan et al, Peptides;2014 54:159–161; [4] A Kamal et al, Immunol Invest 2022;51:899–908.
To cite this abstract in AMA style:
Sundaresan J, Muller I, Lin M, Lafeber F, Welsing P, De Rotte M, Bulatovic-Calasan M, van der Laken C, De Jonge R, Jansen G. Validation of Urotensin-2 mRNA Expression as Marker for Tocilizumab Non-Response in Early Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/validation-of-urotensin-2-mrna-expression-as-marker-for-tocilizumab-non-response-in-early-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-urotensin-2-mrna-expression-as-marker-for-tocilizumab-non-response-in-early-rheumatoid-arthritis/