Session Information
Date: Sunday, November 13, 2022
Title: Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
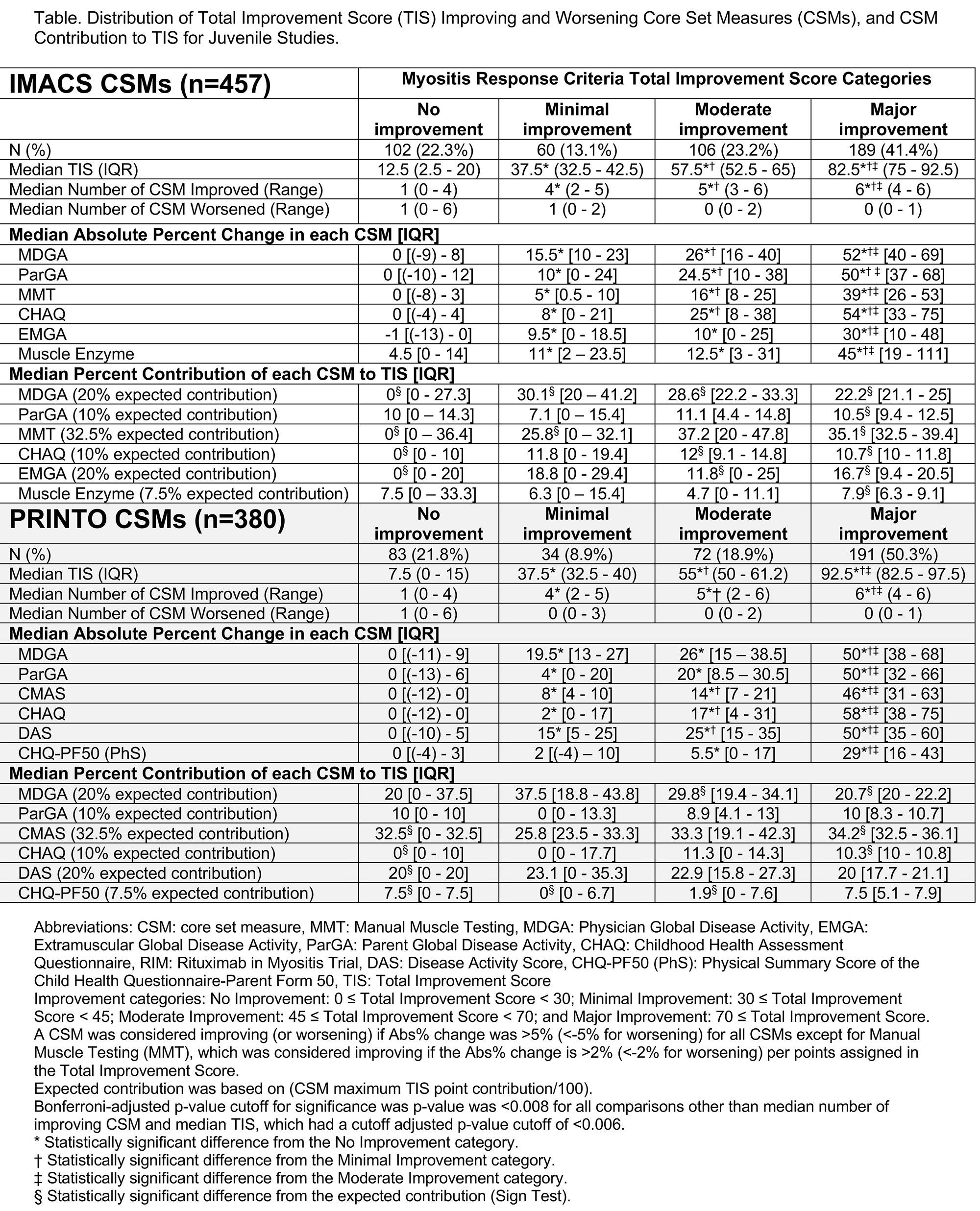
Background/Purpose: Juvenile dermatomyositis (JDM) ACR-EULAR myositis response criteria (MRC) were developed based on absolute % changes in 6 core set measures (CSM) differentially weighted to calculate a Total Improvement Score (TIS). Performance of MRC TIS has not been well-characterized. How changes in International Myositis Assessment & Clinical Studies (IMACS) vs. Paediatric Rheumatology INTernational Trials Organization (PRINTO) CSMs compare, and clinical significance of MRC categories are also unclear. Whether patients can achieve MRC with worsening in individual CSMs, specificity to muscle-related CSMs, and representation of patient-reported (PRO) CSMs are unknown. We aimed to assess the contribution of each CSM to TIS, frequency of muscle CSM improvement among improved patients, representation of PRO in the TIS, relationship of IMACS vs. PRINTO CSMs, frequency of worsening CSMs, and clinical significance of the TIS.
Methods: JDM patients enrolled in the Rituximab in Myositis trial (n=48), PRINTO treatment trial (n=139), and consensus profiles from natural history studies (n=273) were included. TIS and number of improving/worsening CSMs by improvement category were examined. Sign test compared observed vs. expected contribution of each CSM. Frequency of improvement with and without muscle-related CSMs and PRO CSM was calculated. We compared improvement categories by Wilcoxon tests with Bonferroni-adjusted p-values. Change in IMACS and PRINTO CSM and TIS were compared by correlation analysis. Physician rating of change category was compared to MRC improvement categories by weighted Cohen’s Κ test.
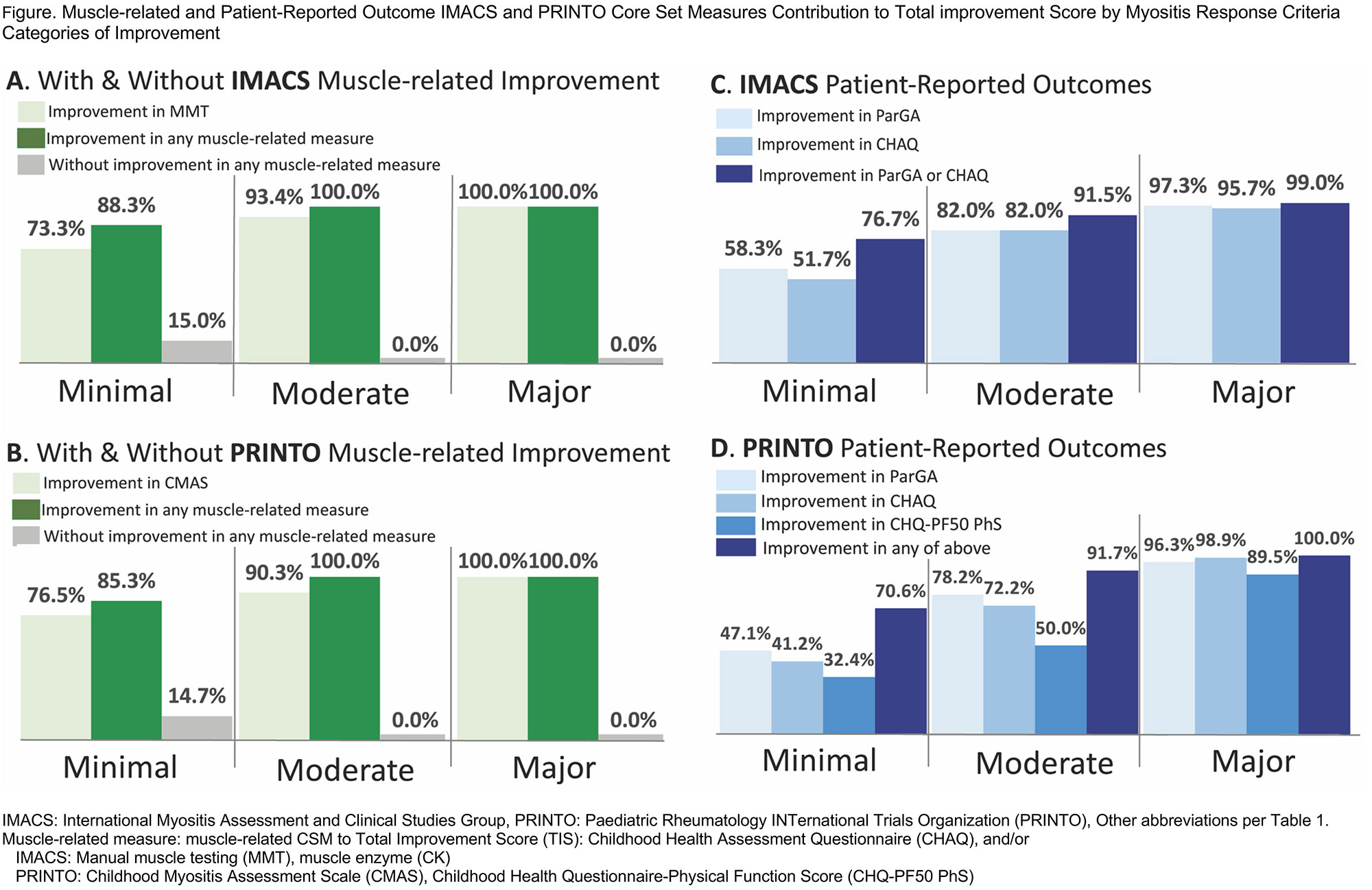
Results: Of 457 total JDM patients with IMACS CSM/ 380 with PRINTO CSM, 13%/ 9% had minimal improvement, 23%/ 19% had moderate improvement, and 41%/ 50% had major improvement using IMACS and PRINTO CSMs (Table). The number of improved CSMs increased with higher MRC improvement categories, as did the percentage change in all CSMs (Table1). Patients with no improvement had a median of 1 CSM worsening, whereas patients with moderate-major improvement had median zero CSM worsening. With minimal improvement, most CSMs contribute to TIS as expected (Table1). Of patients who had at least minimal improvement, 94%/95% had improvement in Manual Muscle Testing or Childhood Myositis Assessment Scale. Of patients with at least minimal improvement, 93%/95% had improvement in at least 1 PRO (IMACS/ PRINTO) (Figure). IMACS and PRINTO CSMs perform similarly for MRC; Changes in CSM for IMACS vs. PRINTO significantly correlated for most measures. Physician-rated categories of change significantly correlated with the TIS improvement categories (weighted Cohen’s κ values 0.5-0.8).
Conclusion: Most JDM patients who improve by the MRC show improvement in muscle-related CSM. MRC improvement also reflects improvement in PRO. Among those who improve by MRC, worsening of CSM is infrequent. Most changes in CSM are significantly correlated between IMACS and PRINTO. MRC TIS categories significantly correlated with clinically meaningful changes by physician assessment. The ACR-EULAR MRC seem robust for the assessment of JDM and perform consistently across these studies.
Acknowledgements: IRP of NIH, NIEHS, NIAMS
To cite this abstract in AMA style:
Kim H, Saygin D, Douglas C, mcgrath j, Wilkerson J, Pistorio a, Reed A, Oddis C, Miller F, Vencovský J, Ruperto N, Aggarwal R, Rider L. Validation of the 2016 ACR/EULAR Myositis Response Criteria in Juvenile Dermatomyositis (JDM) Clinical Trials and Consensus Profiles [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/validation-of-the-2016-acr-eular-myositis-response-criteria-in-juvenile-dermatomyositis-jdm-clinical-trials-and-consensus-profiles/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-the-2016-acr-eular-myositis-response-criteria-in-juvenile-dermatomyositis-jdm-clinical-trials-and-consensus-profiles/