Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Chronic nonbacterial osteomyelitis (CNO) is an autoinflammatory bone disease. It is critical to capture the child’s health-related quality of life impact using validated patient-reported outcome measures. The PROMIS pediatric measures, validated in other pediatric rheumatic diseases, were administered to children with CNO enrolled in the prospective multisite CHronic nonbacterial Osteomyelitis International Registry (CHOIR). Our objective was to assess the convergent and responsive validity of the PROMIS instruments in patients with CNO.
Methods: Children or young adults with CNO were consented and enrolled into CHOIR. Self-reported PROMIS questionnaires of fatigue, pain interference (PI), pain behavior (PB), mobility, upper extremity (UE), physical activity (PA) and strength impact (SI) were administered to patients 8 years and older in English at each clinical visit. Demographic, clinical, and imaging data were prospectively collected. The T score was calculated. External validation surveys were administered to assess patients’ perception of difficulty of use of limb/back/jaw, fatigue, sadness and worry on a 0-10 scale, disease status (inactive, mild, moderate, severe), and status change (unchanged, worsened, improved). Improvement of clinical disease activity score (CDAS) of 2.5 was defined as meaningful change. Descriptive statistics were used for demographic and clinical characteristics. Log-transformed linear mixed effect models with random participant intercepts were performed to assess PROMIS score changes after treatment. Wilcoxon signed-rank test with continuity correction was performed to determine the change of the PROMIS scores among the improved, worsened, and unchanged groups. Spearman rank correlation test was performed to determine the relationship between the PROMIS scores and reported disease status by patient/families.
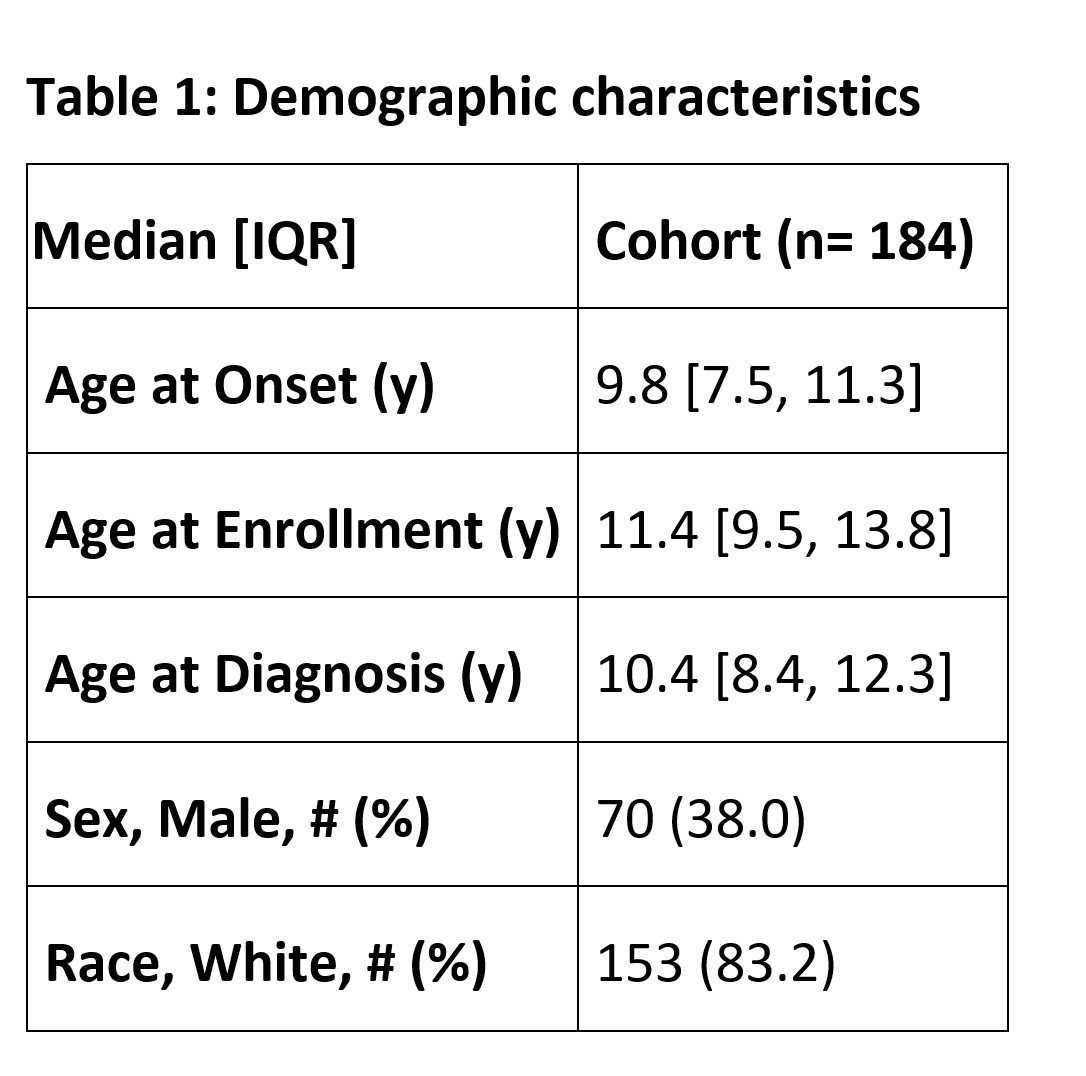
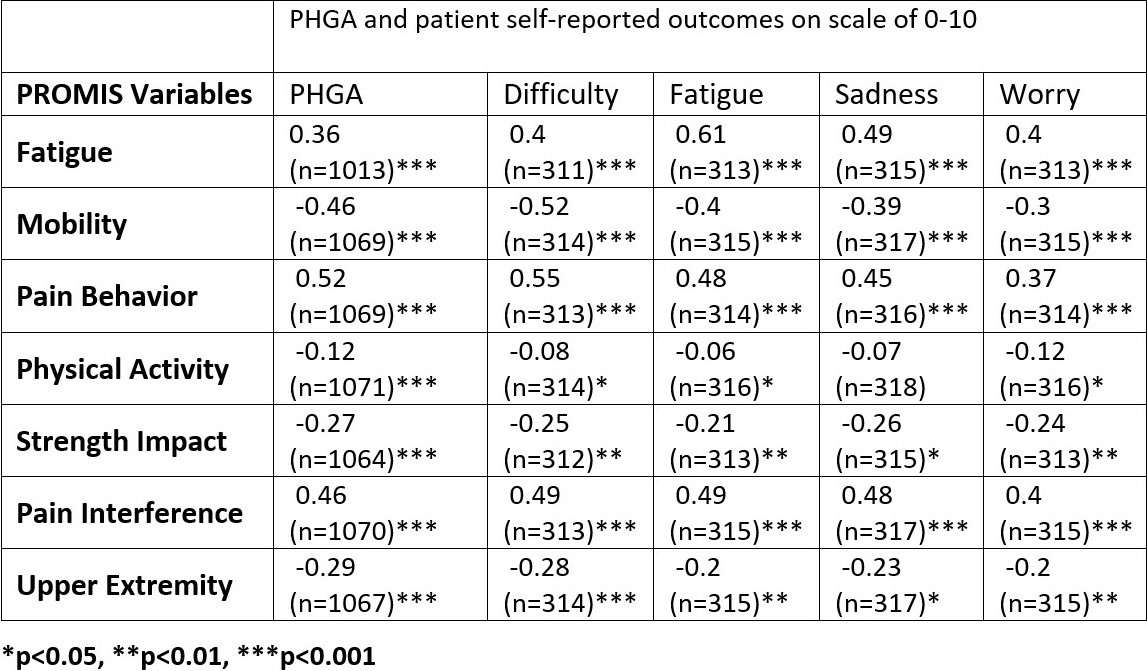
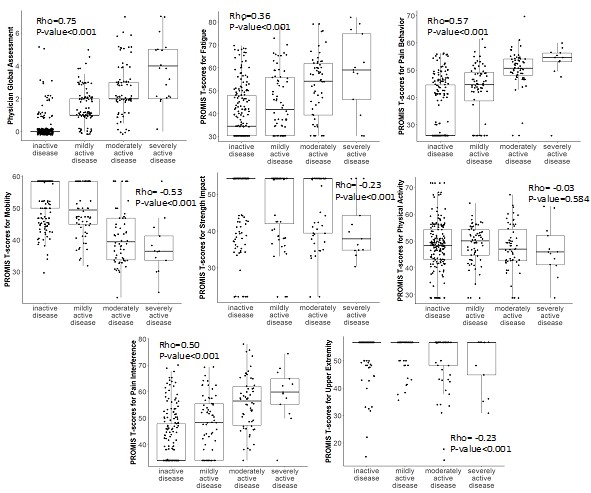
Results: More than 1,000 clinical visits from 184 patients were associated with self-reported PROMIS questionnaire entries in English. Demographic characteristics are shown in Table 1. All PROMIS scores correlated significantly (p< 0.01) with patient reported variables and physician global assessment (PGA) (Table 2). The correlation with functional difficulty and PGA was good (0.4-0.6) for Mobility, PB, and PI. All PROMIS scores, except physical activity, correlated significantly (p< 0.05) with patient reported disease status (Figure 1). The changes of PROMIS scores over time for Mobility, PB, PA, and PI compared to the self-reported status change (unchanged, improved, worsened) was significant (p< 0.05). After effective treatment when clinical disease activity score improved by at least 2.5 points (n=18), the change of PROMIS score from Mobility, PB, PI, UE was significant (p< 0.05).
Conclusion: PROMIS pediatric measures provide valuable information about disease status of children with CNO and correlate well with functional and other psychosocial domains. Mobility, PI, and PB show sensitivity to change after effective treatment or with disease status change (better or worse). These instruments are useful for CNO clinical disease monitoring and research.
To cite this abstract in AMA style:
Eckert M, Wu E, Oliver M, Scheck J, Lapidus S, Akca U, Yasin S, Lenert A, Stern S, insalaco A, Pardeo M, Simonini G, Marrani E, Wang X, Huang B, Kovalick L, Rosenwasser N, Balay-Dustrude E, Casselman G, Adriel L, Klein A, Shao Y, Yang C, Briggs M, Deng E, Hamilton I, Mueller E, Machrone E, Trunnel P, Mosa D, Tucker L, Girschick H, Laxer R, Tiller G, Akikusa J, Hedrich C, Onel K, Dedeoglu F, Twilt M, Ozen S, Ferguson P, Schanberg L, Reeve B, Zhao Y. Validation of Patient-Reported Outcomes Measurement Information System® (PROMIS®) Pediatric Measures for Children with Chronic Nonbacterial Osteomyelitis Using the CHOIR Data [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/validation-of-patient-reported-outcomes-measurement-information-system-promis-pediatric-measures-for-children-with-chronic-nonbacterial-osteomyelitis-using-the-choir-data/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-patient-reported-outcomes-measurement-information-system-promis-pediatric-measures-for-children-with-chronic-nonbacterial-osteomyelitis-using-the-choir-data/