Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Ultrasonography (US) has experienced a rapid evolution in rheumatology. Despite many advantages being shown repeatedly, several barriers persist and stand in the way of a wider use of US in rheumatology, equipment cost being an important one. Hand-held US technology promises to take this cost down substantially. However, before it can be specifically used for rheumatology, its performance needs to be validated against gold-standard devices for key interventions. We aim to test the concurrent validity of a handheld US device versus a gold-standard device to detect characteristic features of healthy and rheumatic joints (i.e., anatomical structures and vascular flow).
Methods: Adult patients with peripheral PsA presenting with at least one tender and swollen joint were included. Each patient had consecutive US examinations using a handheld (Clarius Mobile Health Inc, HD3 L20 and L15 scanners) and a gold standard US device (GE LogicE9) for detecting synovitis, nail disease, and erosions. B mode and power Doppler images were saved for each site and lesion. Every image was given a unique identifier number at the end. Image reading was performed at least 2 weeks after the acquisition of US in all patients. A random order slide show was conducted for scoring, irrespective of the machine used or the anatomical site or patient assessed, to ensure blindness. Scoring was done using previously validated methods. Here we present interim analysis for the first 10 patients to detect synovitis (n=240), nail disease (n=20), and erosions (n=40).
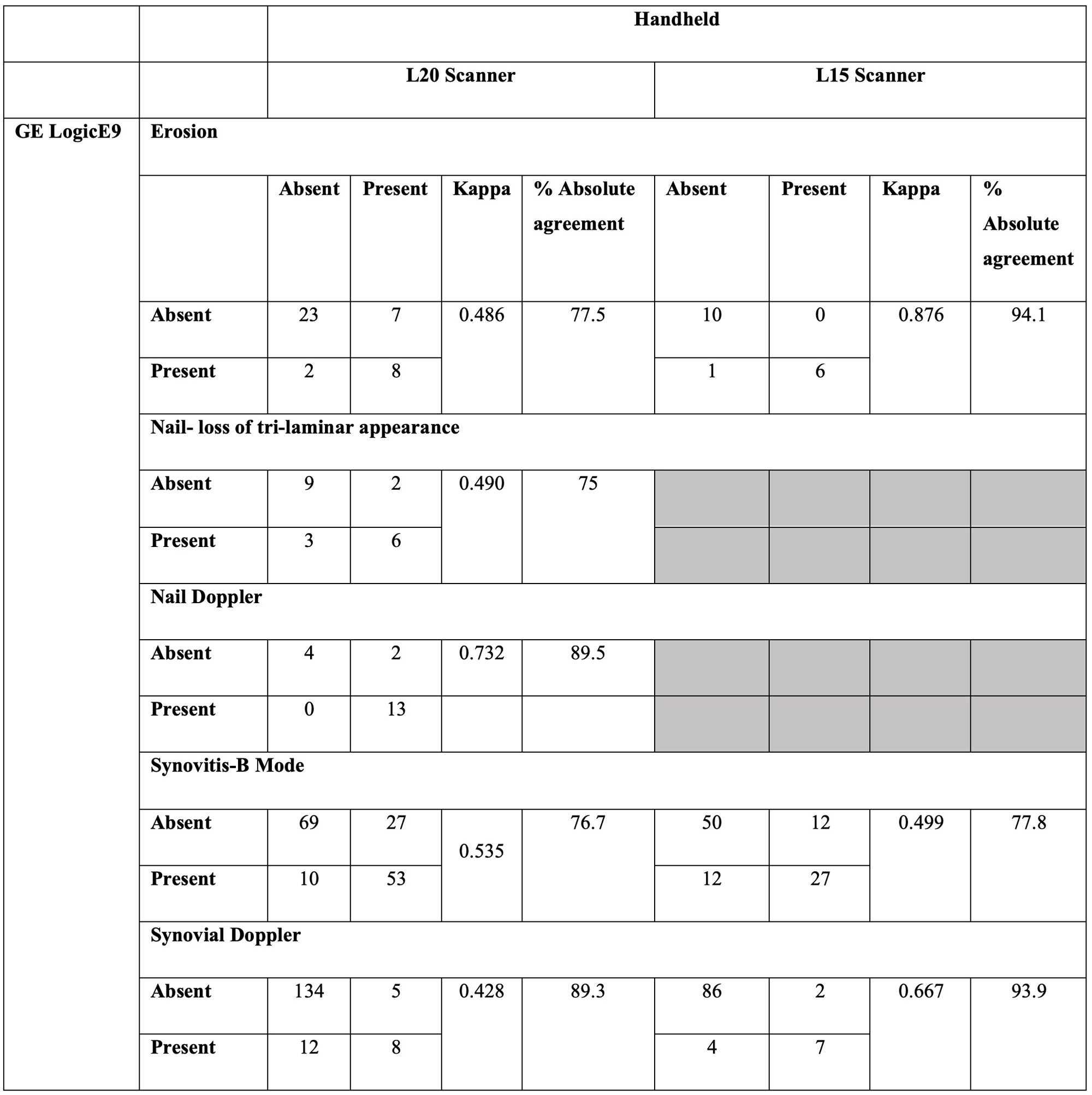
Results: Patients had a mean±SD age of 55.2±8.6, with a median (IQR) disease duration of 7(12) years. 40% were female. On the day of the US, the median (IQR tender joint count was 8.5(18.5) and swollen joint count was 4.5(5.75).At least moderate agreement was observed between the handheld and gold standard devices in all elementary lesions assessed (table). Specifically, both detecting synovitis, the L15 and L20, had a kappa of 0.499 and 0.535 compared to the GE machine, respectively, with absolute agreement rates of 76.7-77.8%. For detecting the intrasynovial Doppler signals, the L15 had a substantial agreement (kappa: 0.667, absolute agreement: 93.9%); and L20 had moderate agreement (kappa: 0.428, absolute agreement: 89.3%). The nail lesions were compared with L20, which showed a moderate agreement to detect the loss of trilaminar appearance (kappa: 0.490, absolute agreement: %75) and substantial agreement to detect nail bed vascularity (kappa: 0.732, absolute agreement: 89.5%). For erosions, there was an almost perfect agreement with the L15 (kappa: 0.876, absolute agreement: 94.1%) and moderate agreement with L20 (kappa: 0.486, absolute agreement: 77.5%). Investigating the individual discrepancies, the L20 seemed to be more sensitive to detecting erosions (fig).
Conclusion: In this interim analysis, the handheld US devices show moderate-substantial agreement to detect synovitis, nail lesions and erosions, and Doppler signals. The final analysis will be conducted after 30 PsA patients are enrolled, and enthesitis will also be analyzed. These interim results encourage testing the use of handheld US devices for wider use.
To cite this abstract in AMA style:
aydin s, acikgoz s, Oguz U, Vieira a, Leclerc p, Shah S, Eder L, S Kaeley G. Validation of Handheld Ultrasound Devices for Point of Care Use in Rheumatology: Interim Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/validation-of-handheld-ultrasound-devices-for-point-of-care-use-in-rheumatology-interim-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-handheld-ultrasound-devices-for-point-of-care-use-in-rheumatology-interim-analysis/