Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The MOBILITY study showed that BASMI often misclassifies spinal mobility, especially in older and taller individuals. To address this, the Corrected AxSpA Metrology Index (CASMI) was developed by adjusting BASMI for age, height, and sex to remove the variability introduced by these factors. This study evaluates CASMI’s psychometric properties and compares them with BASMI in RCTs of axSpA patients treated with b/tsDMARDs.
Methods: RCTs in axSpA patients with complete BASMI component data (lateral spinal flexion, tragus-to-wall distance, intermalleolar distance, cervical rotation, and lumbar flexion) at baseline and the timing of the primary endpoint were included. Data were accessed and analyzed via the data-sharing platform Vivli. Age, sex, and height were used to adjust individual spinal mobility measures. BASMI- and CASMI-linear were calculated, and the following psychometric properties were analyzed: Construct validity: Known-group discrimination (Standardized Mean Difference -SMD) at baseline to distinguish between low and high functional impairment (BASFI < 3 vs >6); Longitudinal construct validity: Standardized Response Mean (SRM), Guyatt’s responsiveness index (GRI), and effect size (ES); Trial discrimination: SMD. For all these psychometric properties, performance was classified as follows: low performance: < 0.5; adequate performance: ≥0.5 and < 0.8; good performance: ≥0.8. Lastly, a sensitivity analysis was conducted in patients with early disease (≤2 years of symptom duration).
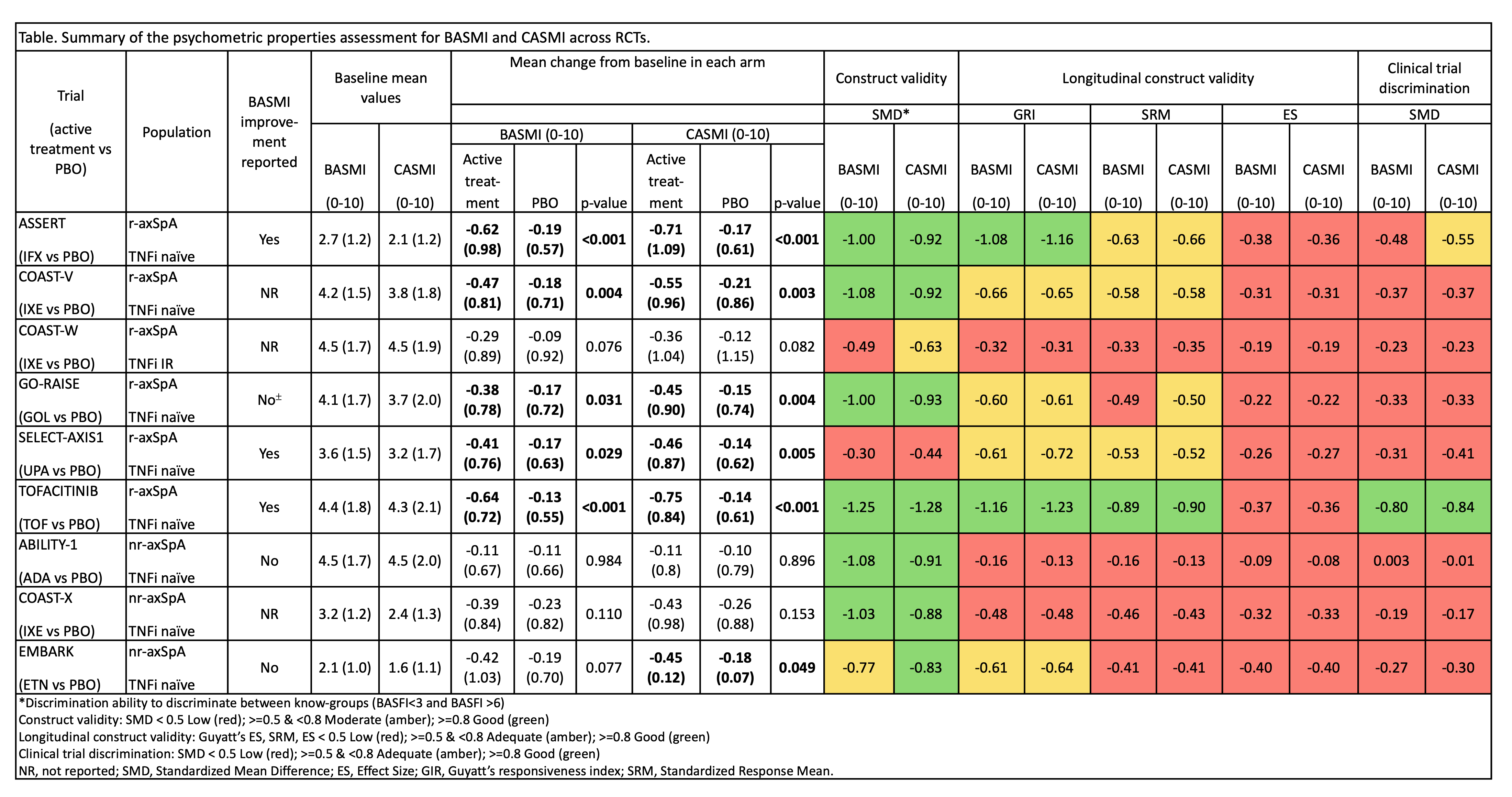
Results: Nine RCTs, all having reached the primary endpoint, with complete BASMI component data and available age, sex, and height information were included. Six involved radiographic axSpA, three non-radiographic axSpA. Only 5 studies included patients with early disease and in varying proportions: 3–48%. In seven of nine trials, CASMI had a lower mean value than BASMI (Table). For construct validity, most trials showed good discriminatory capacity, except COAST-W (TNFi-insuficient response population) and SELECT-AXIS. EMBARK was the only trial where CASMI showed a good discrimination between know-groups, while for BASMI it was only adequate. Regarding longitudinal construct validity, results were inconclusive, showing high variability across measures, but without important differences between BASMI and CASMI. All trials in bDMARD-naïve r-axSpA patients showed significant BASMI/CASMI improvements with active treatment, but trial discrimination was generally low for both, except for CASMI in ASSERT (adequate) and TOFACITINIB (good). In all nr-axSpA studies, trial discrimination was low for both BASMI and CASMI. In analyses restricted to early disease patients, trial discrimination improved numerically but remained low (except for TOFACITINIB, where it remained good), with the exception of SELECT-AXIS, where discrimination became good for BASMI and adequate for CASMI.
Conclusion: While CASMI has been developed to be a more truthful measure than BASMI, both CASMI and BASMI have largely comparable measurement properties. This means that the current adjustments are still insufficient to detect change over time in spinal mobility.
To cite this abstract in AMA style:
capelusnik D, Gardiner P, Boonen A, Nikiphorou E, Ramiro S. Validation of a Corrected Axial Spondyoarthritis Metrology Index in 9 Randomized Clinical Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/validation-of-a-corrected-axial-spondyoarthritis-metrology-index-in-9-randomized-clinical-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-a-corrected-axial-spondyoarthritis-metrology-index-in-9-randomized-clinical-trials/