Session Information
Date: Tuesday, October 28, 2025
Title: (1780–1808) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
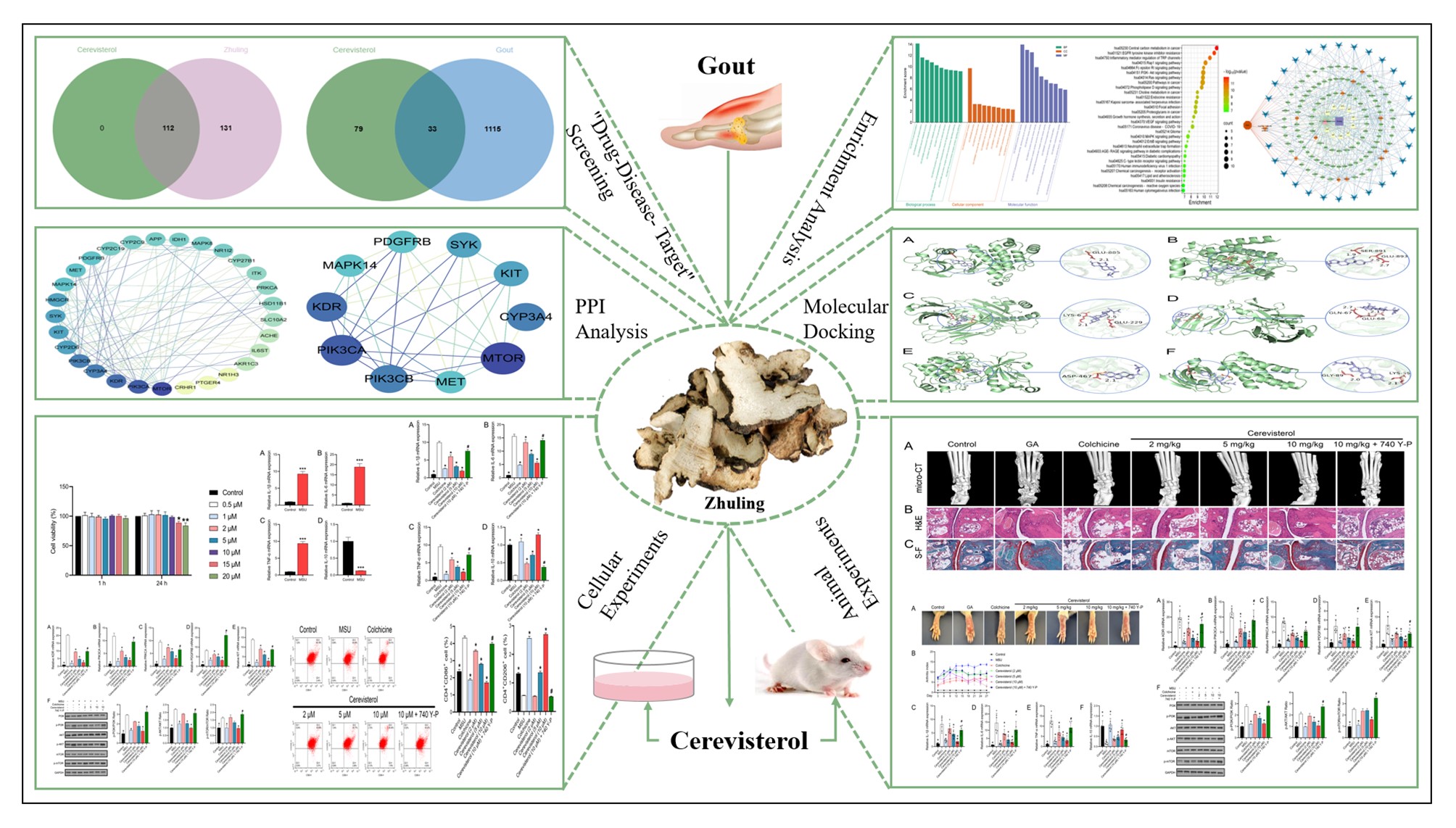
Background/Purpose: Zhuling has traditionally been utilized in the treatment of gouty arthritis (GA). Although its therapeutic benefits are recognized, the molecular mechanisms underlying Zhuling’s action and those of its principal component are still largely uncharted. The purpose of this study was to clarify the molecular mechanisms responsible for the therapeutic benefits of Cerevisterol, a principal component of Zhuling, in the treatment of gout. This was achieved through an integrated approach that combined network pharmacology with in vitro and ex vivo experimental methods.
Methods: We employed the TCMSP database, the Uniprot platform, and SwissTargetPrediction to identify the targets of Zhuling and Cerevisterol. Gout-associated targets were sourced from DrugBank, GeneCards, OMIM, and DisGeNET. Utilizing Cytoscape, we constructed a protein-protein interaction (PPI) network to pinpoint core targets. Pathway enrichment analysis was conducted using Metascape and the Microbiotics platform. Subsequently, we focused on the potential targets and related pathways of Cerevisterol, followed by molecular docking simulations using AutoDockTools and PyMOL. To validate our in silico findings, we established an monosodium urate (MSU)-induced GA cell model using mouse bone marrow-derived macrophages (BMDMs) and evaluated the expression of inflammatory factors, pivotal pathway proteins, and macrophage polarization via RT-qPCR, Western blot, and flow cytometry. We also developed GA mouse models to assess ankle swelling and joint dysfunction indices, and quantified serum inflammatory factors using RT-qPCR. Pathological changes in mouse joints were examined using Micro-CT, H&E staining, and S-F staining, while the impact of Cerevisterol on key pathway proteins was investigated using Western blot.
Results: Network pharmacology and bioinformatics analyses revealed 243 potential targets for Zhuling in gout treatment, among which 112 were potential targets for the treatment of gout by its principal active component, Cerevisterol. Cerevisterol was found to modulate the expression of pro-inflammatory factors such as IL-1β, IL-6, and TNF-α, while upregulating the anti-inflammatory factor IL-10 in MSU-induced GA cells and mouse models. Additionally, Cerevisterol facilitated macrophage polarization towards the M2 phenotype by targeting key molecules like KDR, PIK3CA, PRKCA, PDGFRB, and KIT, Cerevisterol exerted its therapeutic effects by regulating the PI3K/AKT/mTOR signaling pathway and the expression of critical proteins, thereby ameliorating the inflammatory response and pathological changes in GA models.
Conclusion: Our findings suggest that Cerevisterol, a pivotal active ingredient in Zhuling, may exert its therapeutic effects on gout through a multi-target and multi-pathway mechanism. In vitro and in vivo studies corroborate that Cerevisterol can mitigate joint inflammation and pathological alterations in gout by influencing macrophage polarization and the expression of inflammatory mediators through the PI3K/AKT/mTOR pathway. These insights offer a promising avenue for the clinical management of gout.
To cite this abstract in AMA style:
Ma X, Shi M, Chen Q, Zou F, Feng W, Liu Q, Lin C, Li N, Liu X, Xu Q. Validating the Gouty Arthritis Alleviating Effects of Cerevisterol through Integrated In Silico, In Vitro, and In Vivo Studies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/validating-the-gouty-arthritis-alleviating-effects-of-cerevisterol-through-integrated-in-silico-in-vitro-and-in-vivo-studies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validating-the-gouty-arthritis-alleviating-effects-of-cerevisterol-through-integrated-in-silico-in-vitro-and-in-vivo-studies/