Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Minimal important differences (MID), based on within-subject evaluation of attaining an improvement in a continuous outcome such as a pain scale, are important for evaluating the magnitude of treatment effects in randomized clinical trials (RCT) in OA. While designed to assess whether an individual’s improvement achieved a meaningful threshold, MID has often been used to gauge the between treatment-group mean difference in change in the outcome. We propose an alternate analysis, where each subject is evaluated for reaching the MID (yes/no) preserving the subject specific meaning of the MID and then the risk of MID is compared between groups, while allowing for adjustment for baseline values. We examine whether this alternative analysis approach provides complementary information to the between group treatment difference in change in pain.
Methods: As our pain measure, we used WOMAC pain on a 0-100 scale. We evaluated mean differences, and risk differences in subject attainment of MID in 4 completed RCT in knee osteoarthritis with treatment effects ranging from negative/null, to moderate, to strong. The difference between the value at randomization and the value at the end of treatment was the change in WOMAC pain. Based on a systematic review, a reduction of 12 (Devji T. BMJ Open 2017) was used for the MID. Adjusted between treatment group mean differences in change were estimated by analysis of covariance with the outcome of WOMAC pain at the end, by treatment, adjusting for WOMAC pain at baseline and sex. Adjusted risk differences in subject attainment of MID, by treatment, came from marginalized predicted probabilities generated by logistic regression using attainment of MID as the outcome, with treatment, WOMAC pain at baseline, and sex as predictors. 95% confidence intervals were generated by nonparametric bootstrap methods.
Results: Treatment group mean differences ranged from -1.6 to 13.0, and the risk differences ranged from -0.11 to 0.33 (see table). Only 1 RCT had a mean difference above the MID, yet all studies had substantial percentages of subjects with MID improvement. The direction and significance of the mean difference and risk difference results were usually, but not always, concordant. The risk difference analysis made it clear that the control risk for MID varies across RCT in ways that relate to the trial conditions but not necessarily to the treatment effect.
Conclusion: Rather than use the MID to evaluate between treatment group mean differences, we propose using the proportion of subjects attaining MID as a secondary outcome in an RCT evaluating a continuous measure. This preserves the meaning of the MID for which it was validated and allows identifying important information about the subject response to treatment and control in RCT that is not readily apparent from the mean difference.
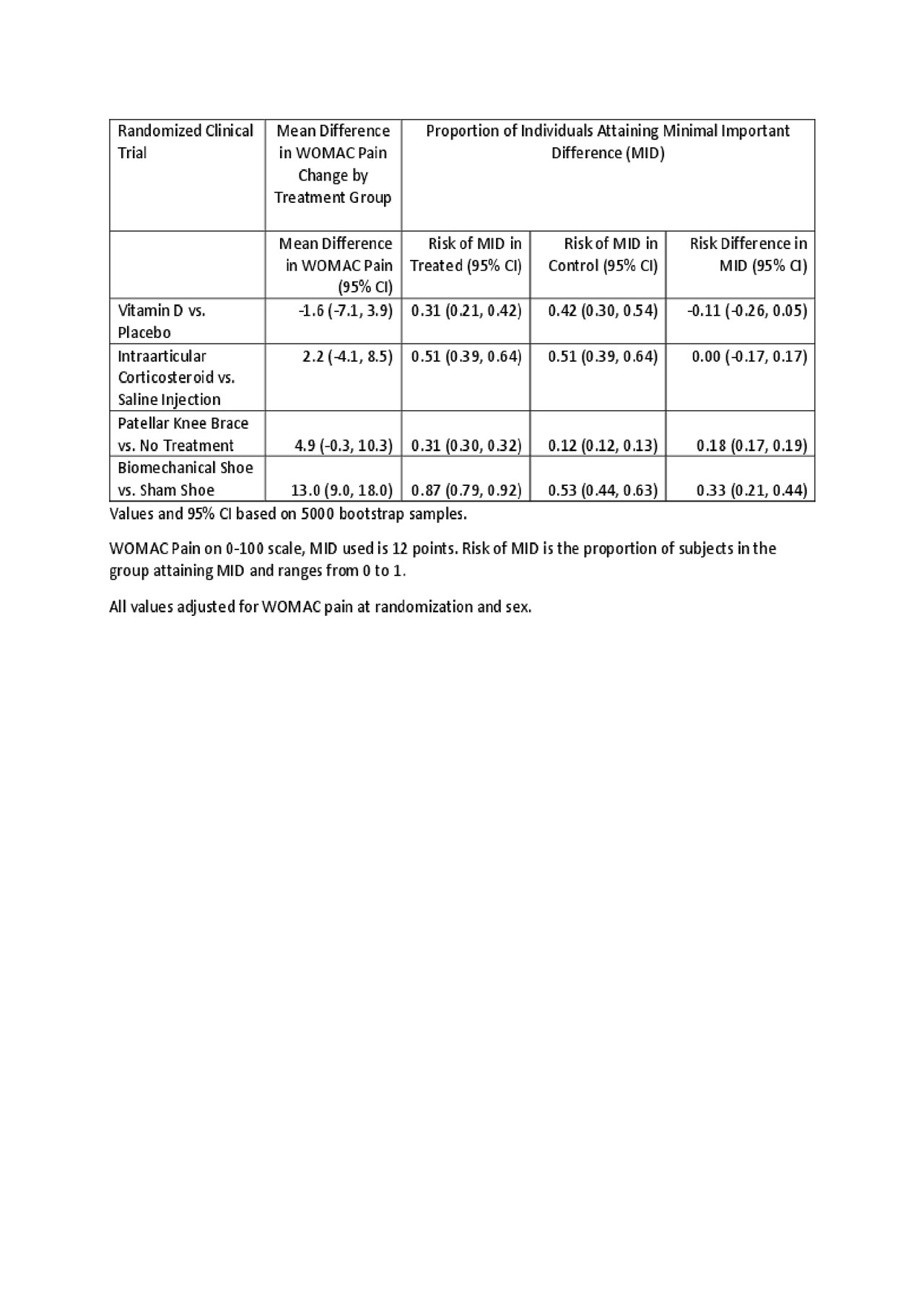
Table for Evaluation of MID for RCT of pain in OA v3
To cite this abstract in AMA style:
Michael L, Parkes M, White D, Reichebach S, McAlindon T, Felson D. Use of Minimal Important Difference (MID) in Randomized Clinical Trials of Pain in Osteoarthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/use-of-minimal-important-difference-mid-in-randomized-clinical-trials-of-pain-in-osteoarthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-minimal-important-difference-mid-in-randomized-clinical-trials-of-pain-in-osteoarthritis/
