Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Safety recommendations for Janus kinase inhibitors (JAKi) issued by the European Medical Agency (EMA) in 2023 could potentially influence prescription patterns for drugs used to treat rheumatoid arthritis (RA), but little is known about the impact of these recommendations in routine clinical care.
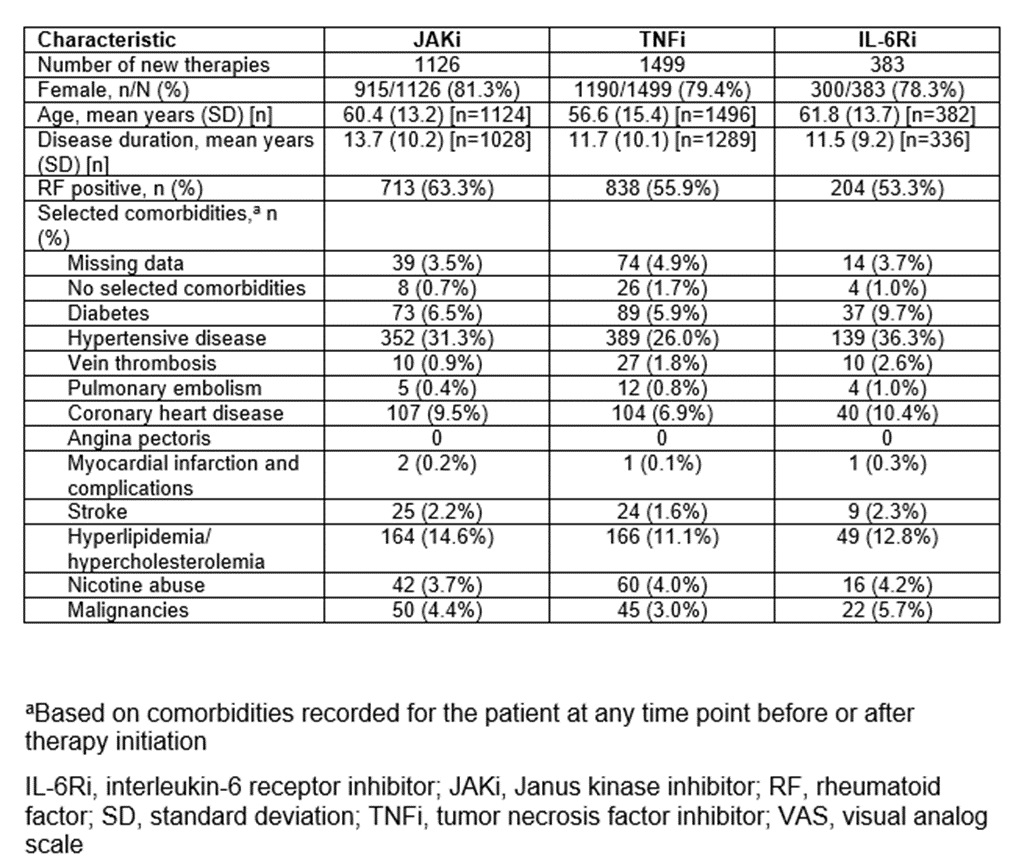
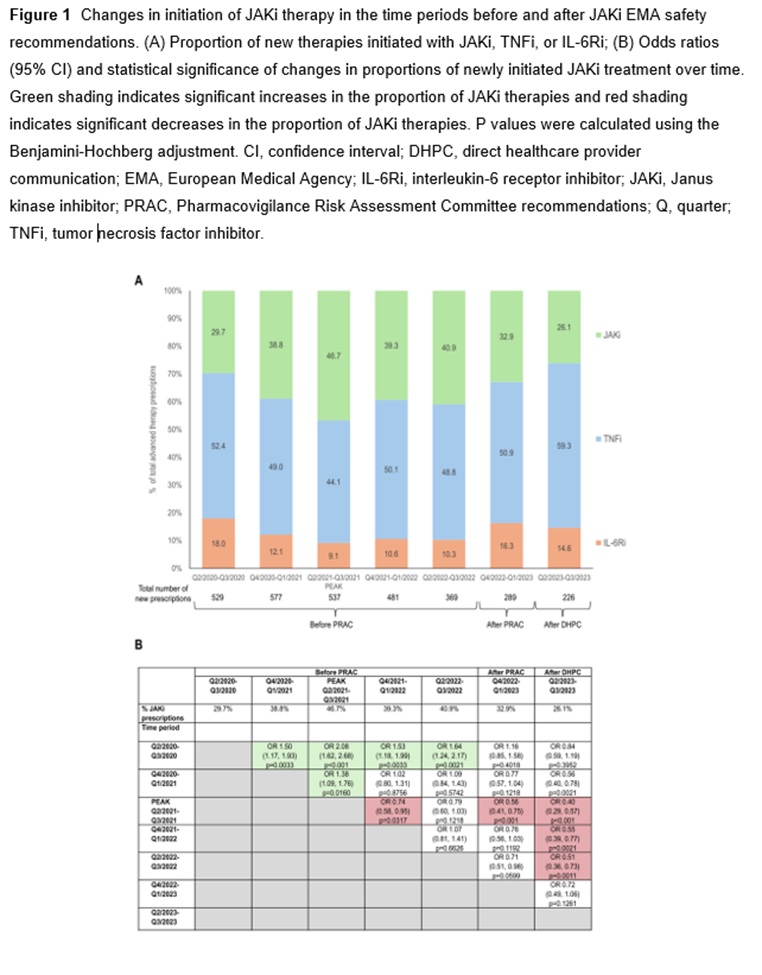
Methods: We retrospectively analyzed the German RHADAR rheumatology database1 for adult patients with RA and documentation of a new therapy with a JAKi, tumour necrosis factor inhibitor (TNFi), or interleukin-6 receptor inhibitor (IL-6Ri). Data were grouped into half-yearly intervals from quarter (Q)2/2020 to Q3/2023. The period from Q4/2022 to Q1/2023 immediately followed the initial EMA endorsement of Pharmacovigilance Risk Assessment Committee (PRAC) 2 recommendations and Q2/2023-Q3/2023 immediately followed the direct healthcare provider communication (DHPC) 3 containing the new safety JAKi recommendations.
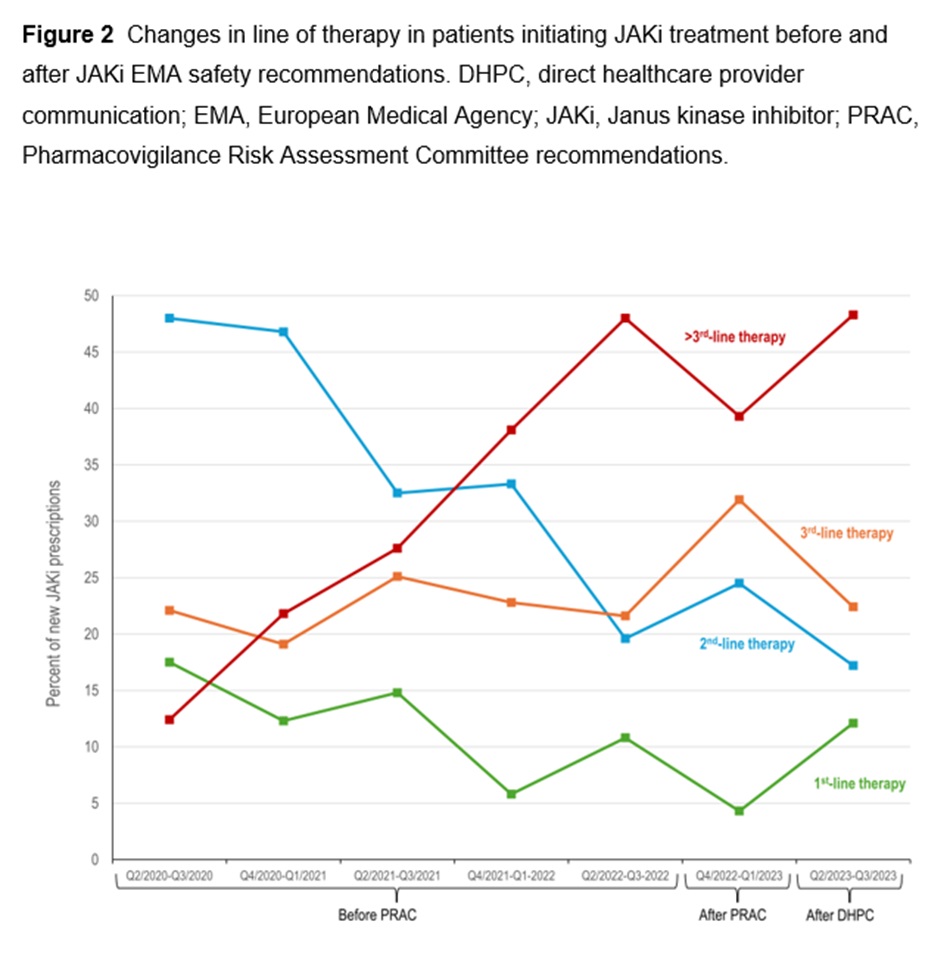
Results: Between April 1, 2020 and September 23, 2023, 3008 newly initiated therapies for TNFi (1499 [49.8%]), JAKi (1126 [37.4%]), and IL-6Ri (383 [12.7%]) were documented by the treating physicians. JAKi were increasingly used in the first two half-year periods (from 29.7% of these therapies in Q2/2020-Q3/2020 to 46.7% in Q2/2021-Q3/2021; odds ratio [OR] 2.08; p< 0.001). The proportion of initiated JAKi therapies decreased significantly after the PRAC recommendations (32.9%; OR vs peak 0.56; p=0.001) and the DHPC letter (26.1%; OR vs peak 0.40; p< 0.001). JAKi were more likely to be used as >3rd-line therapy in later time periods.
Conclusion: This exploratory study suggests that EMA safety recommendations for JAKi influenced treatment patterns of RA patients who received JAKi in Germany. The study showed a significant decrease in the percentage of new JAKi prescriptions and a shift towards using JAKi as later-line therapy post-safety recommendations.
To cite this abstract in AMA style:
Strunz P, Risser L, Englbrecht M, Witte T, Froehlich M, Schmalzing M, Gernert M, Bartz-Bazzanella P, Hueper S, von Der Decken C, Gauler G, Spaethling-Mestekemper S, Kuhn C, Karberg K, Vorbrueggen W, Welcker M, Kleinert S. Use of Janus Kinase Inhibitors Before and After European Medicines Agency Safety Recommendations [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/use-of-janus-kinase-inhibitors-before-and-after-european-medicines-agency-safety-recommendations/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-janus-kinase-inhibitors-before-and-after-european-medicines-agency-safety-recommendations/