Session Information
Date: Sunday, November 7, 2021
Title: Pediatric Rheumatology – Clinical Poster II: SLE, JDM, & Juvenile Scleroderma (0764–0785)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Childhood-onset systemic lupus erythematosus (cSLE) has higher rates of lupus nephritis (LN) than adult-onset SLE, often requiring intensive immunosuppression. This study examined North American practices and preferences for the low-dose EuroLupus cyclophosphamide protocol, as compared to the high-dose National Institute of Health (NIH) cyclophosphamide protocol, to treat LN in cSLE.
Methods: A 35-item web-based survey was distributed to Childhood Arthritis and Rheumatology Research Alliance (CARRA) and Pediatric Nephrology Research Consortium (PNRC) providers. The survey assessed participant demographics, cyclophosphamide prescribing practices, perceptions of EuroLupus protocol, and LN vignette treatment decisions; one vignette was taken from a 2009 CARRA survey and responses were compared. Multivariable logistic regression analyzed provider factors associated with practices and preference for low- versus high-dose cyclophosphamide.
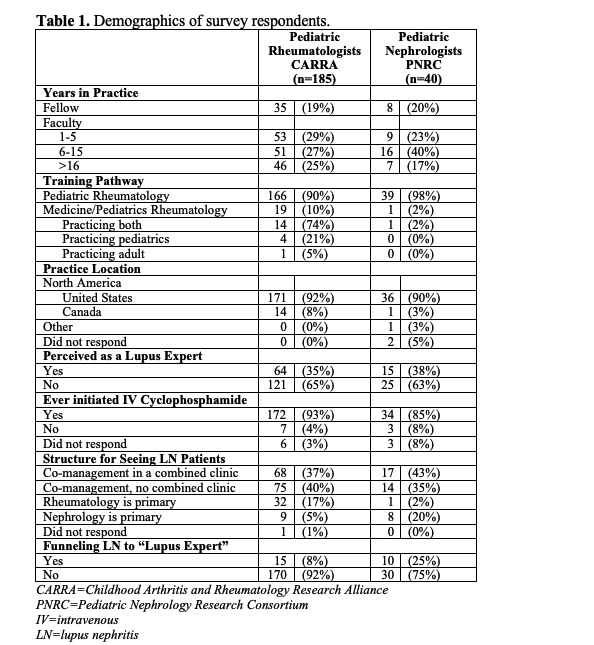
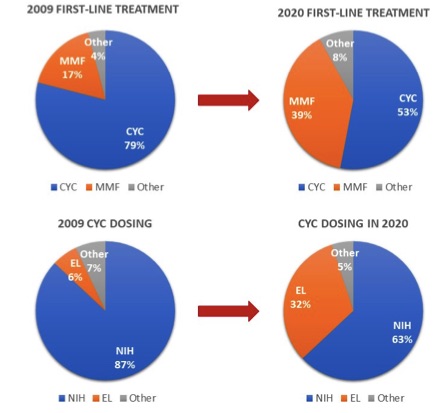
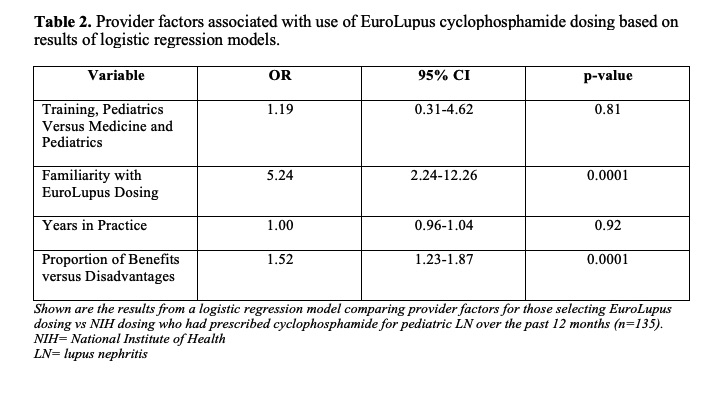
Results: Responses were provided by 185/421 (44%) CARRA physicians (Table 1). Among respondents who prescribed cyclophosphamide for pediatric LN over the past year (n=135), half reported using EuroLupus protocol. When presented the same vignette about an adolescent with class IV LN, 32% of pediatric rheumatologists chose EuroLupus dosing in 2020, versus only 6% in 2009 (Figure 1). Provider factors associated with choosing the low-dose regimen were familiarity with the protocol (OR 4.2, p=0.006) and greater perceived benefit (OR 1.6, p< 0.0001) (Table 2). Forty pediatric nephrologists had similar responses to the pediatric rheumatology providers. Overall, 78% of respondents perceived EuroLupus protocol efficacy to be equivalent to the high-dose protocol in cSLE LN.
Conclusion: Of pediatric specialists who prescribe cyclophosphamide for cSLE LN, more use low-dose cyclophosphamide compared to a decade prior. Nevertheless, familiarity with EuroLupus dosing remains low.
CARRA=Childhood Arthritis and Rheumatology Research Alliance
PNRC=Pediatric Nephrology Research Consortium
IV=intravenous
LN=lupus nephritis
CYC=cyclophosphamide
MMF=mycophenolate mofetil
EL= EuroLupus
NIH= National Institute of Health
Shown are the results from a logistic regression model comparing provider factors for those selecting EuroLupus dosing vs NIH dosing who had prescribed cyclophosphamide for pediatric LN over the past 12 months (n=135).
NIH= National Institute of Health
LN= lupus nephritis
To cite this abstract in AMA style:
Cannon L, Wenderfer S, Lewandowski L, Cooper J, Goilav B, Knight A, Hersh A, Ardoin S, Sadun R. Use of EuroLupus Cyclophosphamide Dosing for the Treatment of Lupus Nephritis in Childhood-Onset Systemic Lupus Erythematosus in North America [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/use-of-eurolupus-cyclophosphamide-dosing-for-the-treatment-of-lupus-nephritis-in-childhood-onset-systemic-lupus-erythematosus-in-north-america/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-eurolupus-cyclophosphamide-dosing-for-the-treatment-of-lupus-nephritis-in-childhood-onset-systemic-lupus-erythematosus-in-north-america/