Session Information
Date: Sunday, October 26, 2025
Title: (0671–0710) Systemic Sclerosis & Related Disorders – Clinical Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: To facilitate clinical trials we previously developed a smartphone app to allow ‘tracking’ of systemic sclerosis (SSc)-related digital ulcers (DUs) and their associated pain and disability, via capture of a combination of photographs and patient reported outcome measures [PROMS]. Members of the patient user group who informed the study felt that the app might be helpful in routine clinical care, as this could provide a means of their clinician viewing the ulcer(s), and assessing treatment response, remotely, thus reducing the need for hospital visits. Our aim was to assess the feasibility of using the app in a clinical setting, and to gauge patient opinion of its benefits, including a newly added lesion feedback function for patients.
Methods: Patients fulfilling the ACR/EULAR 2013 criteria for SSc and who had active DUs were recruited either face-to-face or remotely. Study design throughout was informed by the patient user group. Participants were assessed twice: on study entry (when they downloaded the app on to their own smartphone and were trained in its use) and at the point of DU healing. Participants were asked to image their lesions and record PROMs (including pain score) with the app twice weekly. Specifically for this study a lesion feedback function was added to the app, showing lesion size (measured from the photographs) over time (Figure 1). Feasibility was assessed by the number of patients who elected to participate, by the number of photographs and PROMS recorded via the app, by patient questionnaire feedback (Table 1), and by verbal feedback during patient user group meetings.
Results: Of the 22 patients assessed for eligibility, 10 were recruited: 9 (90%) female, median age (range) 55 (36-74) years, median disease duration (range) 11.6 (1.7-29.7) years. Nine (90%) completed the study. One patient consented for a second time (therefore 10 ‘DU episodes’ were included). Of the 12 patients not recruited, 2 were about to have DU surgery, in 6 the DU had almost healed, and 4 were not interested. The median duration of study participation per DU episode was 39 days (range 18-140). In total, 210 images were returned, median (range) 12 (4-87) per episode from 10 DU episodes. and 101 pain scores returned out of a possible total of 144 (70%). Patient questionnaire feedback is summarised in Table 1: most patients found the app very easy to use, feedback was very helpful, and all were keen to use the app again. One participant required help from someone else to take the photographs, and one patient used a tripod. The patient with most difficulties reported ‘cracked skin’ and limited hand mobility. The 4 patients attending the final user group meeting confirmed feasibility, and made suggestions about ‘next steps’, including feedback on DU size being expressed as percentage change.
Conclusion: 1. Using the smartphone app was feasible for most patients despite the hand function problems of SSc.2. Patients found the feedback function helpful in gauging ‘progress’ of the DU.3. Our findings lend support to wider use of the app in routine clinical practice, thus allowing remote DU monitoring (facilitating early identification of clinical deterioration) and reducing the need for hospital attendance for many patients.
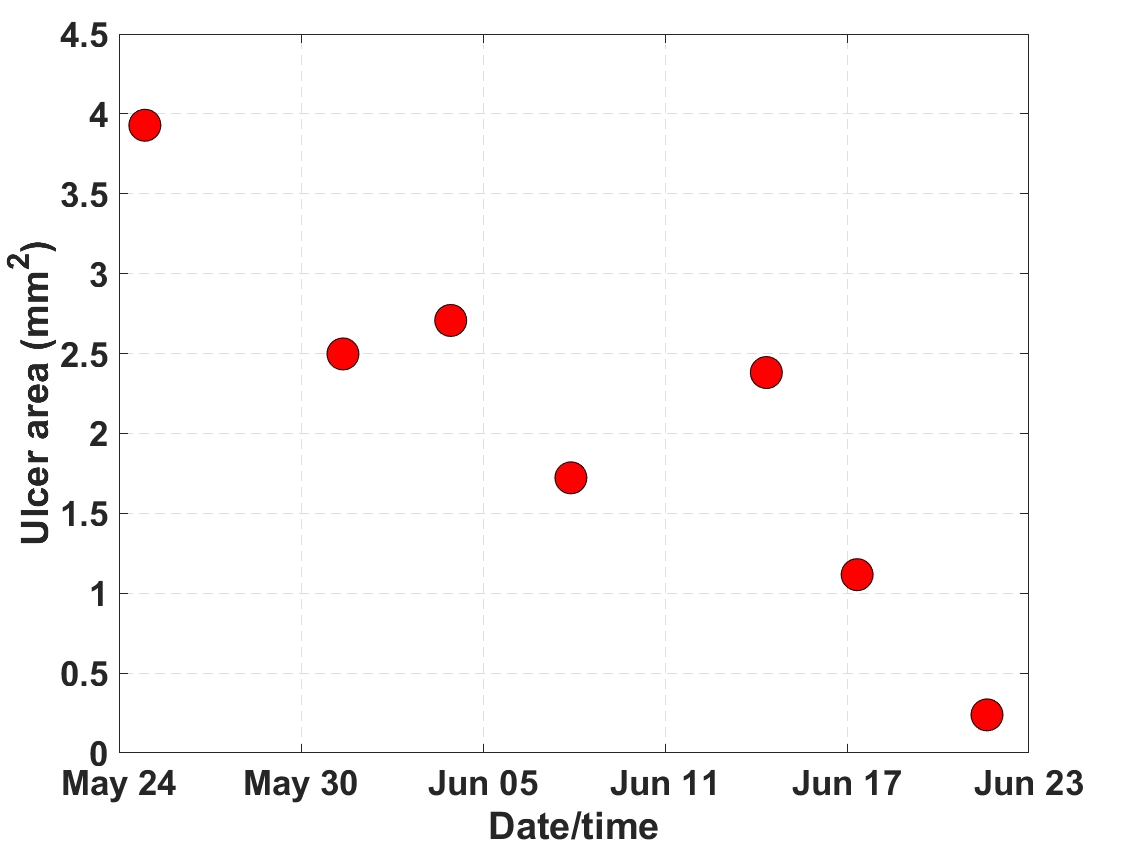 Figure 1 – Example of a lesion feedback graph sent to patients during the study. The ulcer size on the vertical axis is plotted against the date/time stamp of the image from which it was derived
Figure 1 – Example of a lesion feedback graph sent to patients during the study. The ulcer size on the vertical axis is plotted against the date/time stamp of the image from which it was derived
.jpg) Table 1 – Responses to the post-study questionnaire from the 10 ‘episodes’ from the 9 participants who completed the study
Table 1 – Responses to the post-study questionnaire from the 10 ‘episodes’ from the 9 participants who completed the study
To cite this abstract in AMA style:
Herrick A, New P, Dinsdale G, Vail A, Manning J, Hughes M, dixon W, Taylor C, Dickinson M, Murray A. Use of a Smartphone App which Incorporates Feedback to Patients to Monitor Systemic Sclerosis-related Digital Ulcers – a Potential New Tool for Remote Clinical Monitoring [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/use-of-a-smartphone-app-which-incorporates-feedback-to-patients-to-monitor-systemic-sclerosis-related-digital-ulcers-a-potential-new-tool-for-remote-clinical-monitoring/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-a-smartphone-app-which-incorporates-feedback-to-patients-to-monitor-systemic-sclerosis-related-digital-ulcers-a-potential-new-tool-for-remote-clinical-monitoring/
